
Implications of tumor-infiltrating lymphocytes in early-stage triple-negative breast cancer: clinical oncologist perspectives
Introduction
Breast cancer (BC) is the most common malignant tumor and the leading cause of cancer death in women worldwide (1). This heterogeneous disease is caused by several genetic changes in breast epithelial cells, with different clinical manifestations and outcomes (2,3). Gene expression profiling studies have identified at least four categories of BC (4). These molecular categories correlate with immunohistochemical markers of hormone receptor (HR), estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2), with triple-negative subtype defined as the absence of these receptors (5).
The triple-negative BC (TNBC) subtype accounts for 11% to 20% of all BC cases and affects, more commonly, premenopausal patients, women with African ancestry, and carriers of a hereditary mutation in BRCA1/2 genes (6). Characteristically, TNBCs have a biologically aggressive behavior, tend to be larger, more undifferentiated, and with more frequent lymph node involvement at diagnosis (6). This profile is associated to higher recurrence and mortality rates (7).
The immune system plays an essential role in BC initiation and progression (7). The intensity of the tumor’s immune response has been shown to influence the effectiveness of cancer therapy and prognosis (8). In this context, immunotherapy with immune checkpoint inhibitors (ICIs) has emerged as a promising treatment strategy for TNBC. It is currently standard clinical practice in the neoadjuvant setting in combination with chemotherapy, as well as first-line treatment in select patients with metastatic disease (9,10). Despite these recent advances, we still lack biomarkers to help personalize the treatment of BC in general and TNBC in particular. Compared to other subtypes, TNBC is considered more immunogenic and is often associated with higher levels of immune cell infiltration, particularly tumor-infiltrating lymphocytes (TILs) (11). The presence of TILs in TNBC is an independent prognostic biomarker, and it can be potentially used as a predictive biomarker of response to systemic therapies, such as chemotherapy and ICIs (8).
Other several biomarkers are being studied to guide treatment decisions and predict patient outcomes in TNBC, including expression of programmed cell death ligand 1 (PD-L1) or androgen receptors (ARs) and the presence of BRCA mutations (12). Available evidence suggests they may play different roles in early vs. late disease settings. Although TILs have been shown to be a reliable prognostic biomarker, their predictive role for escalation or de-escalation strategies still needs to be better established. Concentrating on early-stage TNBC (eTNBC), this manuscript summarizes essential concepts about the role of the immune infiltrate in BC and the current status and future perspectives of TILs as a prognostic and predictive biomarker.
Current status of systemic therapy in TNBC
Compared to other BC subtypes, eTNBC has high recurrence rates and an unfavorable prognosis (13). This has been attributed not only to its biologically aggressive behavior but also to limited therapeutic options (14). In recent years, outcomes have been improved with the approval of new agents and the use of the neoadjuvant approach as a strategy for individualizing treatment (15).
The treatment of eTNBC is multimodal, including surgery, radiotherapy, and systemic therapy (16). The main goal has been to combine and sequence these different modalities according to the clinical scenario (17). In clinically stage Ia and Ib disease, upfront surgery may be considered appropriate, usually followed by adjuvant chemotherapy, particularly in tumors larger than 5 mm (18). Neoadjuvant therapy is currently the recommended approach in tumors greater than 1 cm and stages II and III (19). Radiotherapy of the breast and regional nodes follows surgery and systemic therapy according to the stage, nodal involvement, and the selected surgical procedure. The response to the neoadjuvant systemic therapy [pathological complete response (pCR) vs. non-pCR] is used to tailor further systemic and locoregional treatment. The objective is to escalate treatment in non-responders or incomplete responders and de-escalate therapy in those with complete response (20). The high chemosensitivity of TNBC confers pCR rates of approximately 40% with the combination of anthracyclines and taxanes (21-23). With the high frequency of homologous recombination defects (HRDs) in these tumors, the addition of carboplatin to neoadjuvant regimens was investigated in phase II and III studies with favorable results consistently increasing pCR rates (24).
Despite a rough start with several phase III trials failing to meet key survival endpoints (25-27) and withdrawn of initially approved agents (atezolizumab), ICIs have been incorporated in the treatment of TNBC. Although initially evaluated in the metastatic setting, early-stage disease represents a promising scenario for the adoption of these agents, since tumor burden is limited and the tumor microenvironment (TME) is less impacted by previous systemic treatments (28).
KEYNOTE-522 is a practice-changing phase III trial that randomized 784 patients with stage II and III eTNBC to receive neoadjuvant chemotherapy (NACT) with concomitant pembrolizumab or placebo (29). The chemotherapy backbone consisted of weekly paclitaxel plus carboplatin followed by anthracycline plus cyclophosphamide every 3 weeks. After surgery, patients continued on adjuvant pembrolizumab or placebo for up to 9 cycles. The study showed a significant increase in the pCR rate (63% vs. 55.6%, P=0.0005) and a prolongation of the event-free survival (EFS) at 3 years [84.5% vs. 76.8%; hazard ratio, 0.63; P=0.0003], the two co-primary endpoints, favoring the group treated with pembrolizumab (29). These results, have established the KEYNOTE-522 regimen as the standard of care for patients with stage II and III eTNBC (19).
However, some caveats and difficulties remain regarding the potential toxicity and the selection of patients who benefit from the addition of programmed cell death 1 (PD-1)-blockade (30). The unique side-effect profile of immunotherapeutic agents is particularly relevant for patients with curable disease. In KEYNOTE-522, almost 13% of patients in the pembrolizumab arm experienced grade 3–5 immune-related adverse events (irAEs), vs. only 1% in the placebo arm (29). Recommendations for a standardized approach to evaluate and treat irAEs have been published and patients should be monitored closely for these events (31).
Importantly, the prognosis of patients who achieve a pCR is highly favorable whether or not they receive immunotherapy (3-year EFS: 92.5% in the control arm vs. 94.4% in the pembrolizumab arm). Although this analysis was exploratory and not powered to make a definitive conclusion, it questions whether adjuvant pembrolizumab adds additional benefits post-pCR (32). The toxicity of adjuvant pembrolizumab was not negligible, with a 6.3% of high-grade irAEs. The OptimICE-PCR study (NCT05812807), is an ongoing clinical trial, that will address the continuation of adjuvant pembrolizumab in patients with pCR. Until the results of this trial are available, a shared decision process should be used to determine whether to continue adjuvant pembrolizumab post-pCR in an individual patient (33).
Notably, a significant proportion of the population treated with the KEYNOTE-522 regimen has residual disease after surgery. This subgroup carries an unfavorable prognosis with 5-year recurrence rates ranging from 30% to 70% depending on the residual cancer burden (RCB) (34). Patients with residual disease had 3-year EFS rates of 56.8% and 67.4% in the control and experimental arms, respectively. In this setting, there is no room for treatment de-escalation and adjuvant pembrolizumab should be prescribed if no contraindication exists. Furthermore, other adjuvant therapies must be considered to improve the outcomes in these patients (35).
The CREATEx trial evaluated the role of capecitabine in patients with residual disease after surgery (36). This study included 910 patients with HER2-negative BC (both HR-positive and TNBC) with non-pCR after NACT. Most of them (80%) received anthracycline and taxane-based regimens (36). Patients were randomized to receive adjuvant endocrine therapy (ET) with or without capecitabine for 6 months. The trial showed positive results in the overall population, with significantly improved disease-free survival (DFS) (74.1% vs. 67.6% at 5 years) and overall survival (OS) (89.2% vs. 83.6%) greater in the capecitabine group compared to the control group, respectively (36). Subgroup analysis demonstrated that most of the OS benefit occurred in the population with TNBC (hazard ratio, 0.58; 95% CI: 0.39–0.87) vs. the HR-positive tumors (hazard ratio, 0.84; 95% CI: 0.57–1.23). This study was the first to confirm that the response to neoadjuvant therapy is discriminating to escalate adjuvant therapy potentially improving survival outcomes (36).
Despite significant advances in our molecular understanding of BC and particularly the heterogeneity of TNBC, there have been very few validated advances in biomarker development to optimize therapy in this context. We mostly continue to treat our patients with a ‘one-size fits all’ approach. Basically, all therapeutic decisions in stage I, II, and III TNBC are based on traditional clinicopathological criteria considering tumor size and nodal status. Importantly, we remain unable to identify who are the patients that will not reach a pCR with neoadjuvant treatment and can only escalate therapy after surgery once the pathological response is known. Therefore, all patients are routinely treated with aggressive and intensive multidrug regimens. In this setting, a predictive biomarker could potentially allow us to de-escalate the neoadjuvant regimen in those patients destined to achieve a pCR and escalate therapy in the others before surgery.
A significant development in this area is the recognition that 10–15% of patients with TNBC carry germline mutations in BRCA1/2 (gBRCA), critical genes for homologous DNA recombination (37). Addressing this particular population of patients, the phase III OlympiA randomized clinical trial evaluated the role of adjuvant treatment with the PARP inhibitor olaparib in high-risk HR-positive, HER2-negative, and TNBC patients with gBRCA (38). In this fantastic collaborative effort, patients were included if they had high-risk characteristics or residual disease after NACT and if they had high-risk characteristics in the adjuvant setting. Patients were randomized to receive olaparib or placebo for 1 year. The study was positive for invasive DFS (hazard ratio, 0.63; 95% CI: 0.50–0.78) and OS (hazard ratio, 0.68; 95% CI: 0.47–0.97; P=0.009) (38). These results establish gBRCA status as a useful biomarker in this setting.
A clear unmet need, there is great interest in developing predictive biomarkers that can help personalize therapeutic decisions in clinical practice. Besides tumor-related biomarkers, the TME role has been increasingly recognized as a critical player influencing tumor evolution and progression, with essential roles in treatment response and resistance development (39). Within the complexity of the TME, immune infiltrates, particularly TILs, have been extensively studied and may be considered as a promising biomarker.
Role of the immune system and the TME in BC
The TME is critically important in tumor development and progression. Cancer cells actively interact with non-malignant cells such as immune system cells, lymphatic vasculature, fibroblasts, and pericytes (Figure 1) (40,41). Regulation of the immune response results from interaction with different classes of T cells such as CD8 T lymphocytes, CD4 T lymphocytes, and regulatory T cells (Tregs) (42).
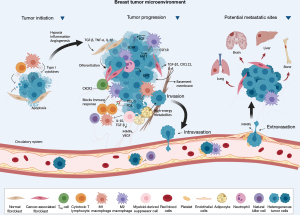
The immune system can recognize and eliminate cancer cells. Still, tumors can evade the immune system and create an immunosuppressive environment, favoring the development and progression of the disease (43). During cancer immunoediting, the host’s immune system shapes tumor fate in three phases through the activation of innate and adaptive immune mechanisms (44). In the first phase, elimination, cancerous cells are destroyed by a competent immune system. Sporadic tumor cells that manage to survive immune destruction may then enter an equilibrium phase where editing occurs. The escape phase represents the third and final phase of the process, where immunologically sculpted tumor cells can grow progressively, become clinically evident, in an established an immunosuppressive TME (44).
In TNBC, the TME significantly influences the malignant behavior and growth of both tumor and surrounding cells. The microenvironment has the ability to reprogram neighboring cells, can counteract the progression of cancer cells, and defining the signaling of cellular pathways, impact the results of therapies. Therefore, the characteristics of the TME define the interaction with the host’s immune system and can affect the response to therapies (45). While specific responses and innate reactions can be harnessed to control TNBC development impeding tumor cells’ initiation, progression, and metastasis, immunosuppressive cells can facilitate immune evasion. The TME in question is closely associated with the characteristic features of TNBC itself, resulting in immune system suppression, evasion of immune detection, and drug resistance (46). The inflammatory infiltration is constituted by all cells with a lymphocytic nature that infiltrate tumor tissues (14). Three TME categories have been defined in different types of tumors: immune desert, comprising tumors devoid of lymphocytes; excluded immune, in which lymphocytes are present only in the peritumoral stroma; and inflamed (“hot”), with high infiltration of T cells (45).
Definition of TILs and standard evaluation
By definition, TILs are mononuclear immune cells that leave the blood and enter the TME, comprising a mixture of cytotoxic and helper T (Th) cells, B cells, macrophages, natural killer cells, and dendritic cells (47). T lymphocytes account for about 75% of TILs (48), and CD8+ cells are abundant in the TNBC microenvironment. Several studies have established a correlation between TILs and TNBC prognosis, indicating that increased expression of CD8+ T lymphocytes is associated with better clinical outcomes (49,50).
The presence of CD3+ T cells is frequently observed in the TME representing the mature T cell population with co-differentiated antigens on their surface, serving as markers for total T lymphocytes within the tissue. Further characterization of infiltrating cell population reveals distinct subsets, including CD8+ T lymphocytes, CD4+ Th lymphocytes, and CD4+ Tregs.
The CD4+ Th lymphocytes can be categorized into Th1 and Th2 subtypes based on their secretion of cytokines and participate in cellular and humoral immunity, respectively. CD4+ Th lymphocytes assist in CD8+ T lymphocyte-mediated cell killing, actively contributing to the tumor immune response. On the other hand, Tregs, which constitute 10% of all CD4+ T lymphocytes in the peripheral blood of healthy individuals, can increase to 30–50% within tumor lesions, inhibit the activation of CD8+ T and CD4+ T lymphocytes, playing a crucial role in immune suppression and angiogenesis and potentially hampering the body’s anti-tumor immune response. This significant accumulation of immunosuppressive Treg subsets, can include high infiltration of forkhead box P3 (FOXP3+) cells (46). These FOXP3+ Tregs can suppress immune responses against self-antigens, hinder anti-tumor immunity, and are a prognostic indicator of poor outcome (51).
Within the TNBC TME, TILs should be assessed by analyzing immune cells’ presence, density, and distribution within the tumor. This can be done through various techniques, including histological examination, immunohistochemistry, flow cytometry, and gene expression profiling (52). The TILs Working Group is a cooperative group of researchers who has developed guidelines to standardize and allow greater reproducibility of TILs assessment in BC (53). In summary, the general rules for evaluating TILs range from pre-analytical guidelines, such as slide preparation and fixation (the ideal thickness of the slide should be 4 to 5 µm of tissue fixed in formalin and embedded in paraffin) to analytical issues (53). TILs should be evaluated within the limits of the invasive component of a tumor. However, only the stromal component should be considered and importantly, areas occupied by carcinoma cells should not be included in the total surface area evaluated. TILs in areas with crushing artifacts, regressive hyalinization, and necrosis and those at a previous biopsy site should be excluded from consideration. The percentage of TILs present in a given tumor should be calculated by dividing the area occupied by mononuclear inflammatory cells by the total area of the tumoral stroma. The value should be given as a percentage and is a continuous variable. If the percentage of TILs is uncertain, the case should be discussed with a second pathologist (53). Standardization of the evaluation allows for greater reproducibility and agreement and, therefore, facilitates the development of studies evaluating TILs as a potential biomarker of impact in clinical practice.
Prevalence of TILs in eTNBC
The molecular subtype of BC impacts the interaction with the immune system. TNBC is more often infiltrated by TILs than luminal tumors (45). Although we should value the recommendations from the International TILs Working Group, there is no consensus on the ideal cutoff point for determining high and low TILs (53). The German Breast Cancer Group conducted a study evaluating the predictive and prognostic value of TILs and defined three groups: low (0–10%), intermediate (11–59%), and high (≥60%) TILs (45). Determining a specific cut-off point is an important and complex issue, as the impact of TILs expression, both on the prognosis and on the predictive importance of this biomarker, appears to be linear (45).
Some studies use the term lymphocytic predominantly BC (LPBC) to define tumors that have a TIL density greater than or equal to 50–60% (54-59), considered as having “high TILs”. Other studies have used different cutoff points to assess the impact of TILs. It is important to emphasize that although TNBC is considered the most immunogenic among BC subtypes, most early TNBC have a low or intermediate density of TILs (60). Table 1 summarizes some recent trials assessing the prevalence of stromal TILs (sTILs) in eTNBC. Not all studies cited follow the evaluation rules defined by the TILs working group, which hampers comparative analyses. Despite this, the studies agree regarding the prevalence of sTILs in the studied populations.
Table 1
| Author, year, reference | Number of TNBC patients | Cutoff to define “high TILs” | Prevalence |
|---|---|---|---|
| Kimura et al., 2023, (61) | 54 | 50% | High: 27.80% |
| Low: 62.20% | |||
| Agarwal et al., 2023, (62) | 108 | 60% | High (≥60%): 15.7% |
| Intermediate (11–59%): 29.9% | |||
| Low (≤10%): 34.4% | |||
| Candelaria et al., 2022, (63) | 284 | 20% | High: 32% |
| Low: 68% | |||
| de Jong et al., 2022, (64) | 441 | 75% | High (≥75%): 21% |
| Intermediate (31–74%): 27% | |||
| Low (≤30%): 52% | |||
| Stecklein et al., 2023, (65) | 110 | 20% | High: 51% |
| Low: 49% | |||
| Sharma et al., 2022, (66) | 117 | 30% | High: 47% |
| Low: 53% | |||
| Gluz et al., 2022, (67) | 336 | 60% | High: 13% |
| >10%: 33.8% | |||
| ≤10%: 66.2% | |||
| Loibl et al., 2019, (68) | 174 | 60% | High (≥60%): 14% |
| Intermediate (11–59%): 48% | |||
| Low (≤10%): 38% | |||
| Denkert et al., 2018, (55) | 906 | 60% | LPBC: 30% |
| Galvez et al., 2018, (69) | 86 | 50% | High: 50% |
| O’Loughlin et al., 2018, (70) | 75 | 50% | LPBC: 12% |
| Adams et al., 2014, (56) | 481 | 50% | LPBC (≥50%): 5% |
| Intermediate (11–49%): 75% | |||
| Low (≤10%): 20% | |||
| Pruneri et al., 2016, (71) | 897 | 50% | LPBC: 21.9% |
| Pruneri et al., 2016, (57) | 647 | 50% | LPBC: 18% |
| Tian et al., 2016, (58) | 425 | 50% | LPBC: 3.5% |
| Denkert et al., 2015, (72) | 314 | 60% | LPBC: 28.3% |
| Denkert et al., 2010, (54) | 1,058 | 60% | High: 61% |
TILs, tumor-infiltrating lymphocytes; eTNBC, early-stage TNBC; TNBC, triple-negative breast cancer; LPBC, lymphocytic predominantly breast cancer.
TILs as a prognostic biomarker in eTNBC
Evidence of the impact of TILs as a prognostic biomarker highlights the importance of the immune response and the role of the TME in tumor development and control. A meta-analysis of approximately 13,100 patients demonstrated an association between a high density of TILs and better prognosis in BC, including better DFS and OS (73). The systemic immune response, defined by the TILs score and the systemic inflammation index (determined by platelet × neutrophil/lymphocyte) has also been correlated with survival outcomes (74). It is important to highlight the dynamic characteristic of TILs density during the evolution of the disease. The cellular population in the TME is impacted by systemic treatment. An increase in TILs during neoadjuvant treatment appears to be associated with better outcomes in TNBC. The survival benefit of higher levels of infiltration was demonstrated in a meta-analysis that analyzed studies that performed paired analyses of TILs density before and after NACT (75). The NeoTRIP study also demonstrated an increase in TILs after 1 cycle of neoadjuvant systemic therapy (76). The prognostic value was assessed in both initial and residual diseases after neoadjuvant therapy, where a greater lymphocytic infiltrate also demonstrated an association with favorable outcomes (77). Importantly, better definition of the international standards for assessment of TILs in residual disease and in surgical specimens with pCR is needed to validate the potential role of dynamic changes in TILs after neoadjuvant therapy.
As described in Table 2, several studies have evaluated the prognostic role of the presence and density of TILs on survival outcomes of patients with eTNBC. Although some studies were carried out prior to the standardization of TILs assessment, the positive correlation between TILs and better prognosis has been consistent. The findings consistently show a linear behavior, where for each 10% increase in the density of sTILs, there is a reduction in the risk of the event (recurrence or death) in the order of 5% to 20%. Two studies (57,64) describe excellent survival outcomes, with 10-year OS rates of around 95% in patients with eTNBC and high TILs. Loi et al. conducted a pooled analysis of 2,148 patients undergoing adjuvant treatment for TNBC (83). The study showed that stage II high TILs patients had better prognosis and improved OS compared to clinical stage I with lower infiltration of TILs (79). This important and surprising result underscores the concept that biological characteristics should be better understood and further evaluated for risk classification of BC patients. They suggest that TILs can be superior to the anatomical staging system used dogmatically in breast oncology for decades.
Table 2
| Author, year, reference | N | Outcomes | Results |
|---|---|---|---|
| Agarwal et al., 2023, (62) | 108 | DFS and OS | High sTIL associated with better DFS and OS |
| de Jong et al., 2022, (64) | 441 | OS and DRFS | Each 10% sTIL decrease risk of death in 19% (hazard ratio: 0.81) |
| De Jong et al., 2020, (78) | 481 | OS and DRFS | Each 10% sTIL decrease risk of event in 17% (hazard ratio: 0.83) |
| Loi et al., 2022, (79) | 2,148 | IDFS, DDFS, and OS | Each 10% sTIL decrease risk of event in 13–17% (IDFS, hazard ratio: 0.87; DDFS, hazard ratio: 0.83; OS, hazard ratio: 0.84) |
| Gluz et al., 2022, (67) | 336 | IDFS, DDFS, and OS | High sTIL associated with better IDFS, DDFS, and OS |
| Gao et al., 2020, (59) | 18,170 | DFS and OS | High sTIL associated with increased DFS (hazard ratio: 0.907) and OS (hazard ratio: 0.869) |
| Park et al., 2019, (80) | 476 | IDFS, DDFS, and OS | High sTIL stage I: 5-year IDFS 91%; 5-year DDFS 97%; 5-year OS 98% |
| Denkert et al., 2018, (55) | 906 | DFS and OS | Each 10% sTIL decrease risk of event in 7–8% (DFS, hazard ratio: 0.93; OS, hazard ratio: 0.92) |
| Leon-Ferre et al., 2018, (81) | 605 | IDFS and OS | Lower TIL associated with worse IDFS and OS |
| Pruneri et al., 2016, (71) | 897 | DFS, DDFS, and OS | Each 10% TIL better DFS, DDFS, and OS |
| Pruneri et al., 2016, (57) | 647 | BCFI, DFS, DRFI, and OS | Each 10% TILs decrease risk of event 11–17% (BCFI, hazard ratio: 0.87; DFS, hazard ratio: 0.89; DRFI, hazard ratio: 0.84; OS, hazard ratio: 0.83) |
| Tian et al., 2016, (58) | 425 | DFS, DDFS, and OS | Each 10% sTILs decrease risk of event 5% (recurrence or death) |
| Dieci et al., 2015, (82) | 199 | OS | OS 10-year: HT 89%, LT 68% |
| Adams et al., 2014, (56) | 481 | DFS, OS, and DRFI | Each 10% sTIL decrease risk of event in 14% |
| Dieci et al., 2014, (77) | 278 | MFS and OS | Each 10% sTIL decrease risk of event in 21% (mets or death) |
| Loi et al., 2013, (83) | 256 | DFS and OS | Each 10% sTIL decrease risk of event 15–17% |
TILs, tumor-infiltrating lymphocytes; eTNBC, early-stage TNBC; TNBC, triple-negative breast cancer; DFS, disease-free survival; OS, overall survival; sTIL, stromal TIL; DRFS, distant relapse-free survival; IDFS, invasive DFS; DDFS, distant DFS; BCFI, breast cancer-free interval; DRFI, distant recurrence-free interval; HT, high TILs; LT, low TILs; MFS, metastasis-free survival.
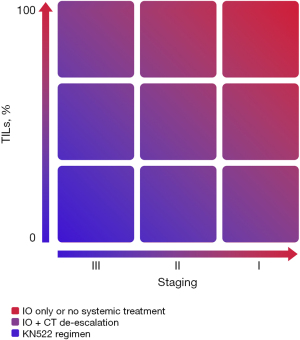
The prognostic correlation was also evident in a cohort of patients who did not undergo chemotherapy treatment. Park et al., evaluated approximately 480 patients with early disease, exclusively submitted to local treatment and not exposed to any systemic therapy, and showed an impressive long-term OS in those patients with a high percentage of sTIL (≥30%) (80). In the same context, a Dutch cohort evaluated more than 400 patients aged <40 years and with node-negative disease, not submitted to any systemic treatment. Patients with high TILs (more than 75%) had better prognosis and a reduced risk of distant recurrence and death than those with low TILs (78). The 15-year incidence of metastases or death of was only 2.1%, and compared favorably to the 38% observed in the low TIL cohort (those with less than 30%) (64). These data raise the question of whether TILs could be used in clinical practice to guide de-escalation strategies for patients with early disease and “high TILs”. This provocative concept is summarized in Figure 2 (84), although it is important to emphasize that it requires further validation.
In addition to the quantitative analysis of TILs, evaluating the subtype of T cells in the TME is extremely relevant and has been addressed in several studies. As mentioned, different T cell subtypes can play an activating or suppressing role in carcinogenesis, progression and treatment response, depending on the cell subpopulation identified (85). A TME with predominantly CD8+ and CD4+ T cells infiltration has been correlated with good prognosis in various cancer types (48,50,86). In contrast, a greater infiltration with Tregs (associated with immunosuppression) determines a worse prognosis and associates with more aggressive tumors, unfavorable clinicopathological characteristics (high tumor grade, high-risk disease), late relapses, and worse survival (87-90). The correlation between infiltrating Treg and CD8+ T lymphocytes has been shown to have prognostic and predictive significance, depending on the location and density of each subpopulation (88). Although the prognostic impact is evident and consistent in several studies, so far there is no support in the literature for using the TILs as a biomarker to omit or escalate systemic treatment (49).
TILs as a predictive biomarker in eTNBC
The neoadjuvant approach is considered an optimal scenario to evaluate the interaction between anticancer drugs and tumor response (88). Studies have shown that higher levels of TILs within the TME are associated with a greater likelihood of achieving a pCR following NACT in eTNBC. A pCR is correlated with better long-term outcomes and survival rates. Studies have elicited a linear relationship between the level of TILs and clinical and pathological response rates in TNBC patients submitted to NACT, as summarized in Table 3. The addition of platinum use in the neoadjuvant regime was evaluated in the GeparSixto study, which included 314 patients with eTNBC. The study demonstrated a positive association for pCR in patients with LPBC. In this study, the TNBC patients with LPBC treated with a platinum salt had a pCR rate of 75% and the chance of a pCR was 3.71-fold compared to that of non-LPBC population (72). Another study demonstrated numerically higher pCR, but no statistical difference, between platinum-based and non-platinum-based regimens in the high TIL subgroup (91). These data suggest that the subpopulations of T cells involved in the inflammatory infiltrate in the TME may have an impact on the response to treatment. This is in line with the rationale for an immunomodulatory function of the immune infiltrate and with the prognostic impact we previously discussed. Furthermore, available data also suggests that higher CD8+/FOXP3+ ratios and high levels of CD4+ and CD8+ TILs are associated with a greater probability of obtaining pCR in eTNBC (88,96). In addition, the presence of TILs both at presentation and in the residual disease after exposure to systemic treatment is associated with a favorable outcome and seems to be inversely related to the activation of the cell proliferation RAS/MAPK pathway (88).
Table 3
| Author, year, reference | Number of TNBC | High TILs cutoff | pCR high TILs | pCR low TILs |
|---|---|---|---|---|
| Abdullaeva et al., 2023, (91) | 132 | 40% | 63.3% | 46.1% |
| Agarwal et al., 2023, (62) | 108 | 60% | 52.90% | 21.10% |
| Sharma et al., 2022, (66) | 117 | 30% | 78% | 45% |
| Gluz et al., 2022, (67) | 336 | 60% | 59.30% | 29% |
| Bianchini et al., 2020, (76) | 260 | 40% | 71% | – |
| Schmid et al., 2020, (92) | 60 | 40% | 74–78% | – |
| Denkert et al., 2018, (55) | 906 | 60% | 50% | 31% |
| Loibl et al., 2019, (68) | 174 | 60% | OR 3.09 HT × IT | |
| Herrero-Vicent et al., 2017, (93) | 164 | 40% | 88% | 9% |
| Tomioka et al., 2018, (94) | 32 | 30% | 30% | 21% |
| Hida et al., 2016, (95) | 48 | 50% | 63% | 17% |
| Denkert et al., 2015, (72) | 314 | 60% | 75% | – |
| Denkert et al., 2010, (54) | 1,058 | 60% | 40% | 7% |
eTNBC, early-stage TNBC; TNBC, triple-negative breast cancer; TILs, tumor-infiltrating lymphocytes; pCR, pathological complete response; OR, odds ratio; HT, high TILs; IT, intermediate TILs.
The impact of TILs has also been evaluated in tumors treated with immunotherapy in combination with NACT. A correlation between sTILs and pCR has been demonstrated, suggesting a predictive value for TILs in response to ICI. These data must be cautiously considered, as they often result from exploratory analyses (97). Studies evaluating the use of pembrolizumab in the context of neoadjuvant therapy in the KEYNOTE-173 (92), I-SPY (98), and NeoPACT (66) studies, explored the association of high TILs and pCR. An analysis of the NeoPACT study hypothesizes that lymphocyte-dependent response mechanisms dominate the therapeutic response in sTIL-high tumors. In contrast, in sTIL-low tumors, the response may be more related to the proliferation index, identified by proliferation gene expression signatures, with no impact from the TILs population (65). Studies evaluating other checkpoint inhibitors, such as Atezolizumab and Durvalumab, also demonstrate a positive association between TILs and pCR rates. The NeoTRIP study found a pCR rate of 74.1% in the population with TILs >40% before cycle 2 (76). The NeoMONO study evaluated biomarkers that can predict early response or resistance to immunotherapy in a cohort of 101 patients. Preliminary data showed that high TILs at the onset or increased TILs after 2 weeks of atezolizumab monotherapy are associated with an increased likelihood of pCR, with rates around 85% (99). The GeparNuevo study evaluated the combination of Durvalumab or placebo with chemotherapy and stratified patients by TIL density. The population with high TILs (>60%) had higher pCR rates in both groups (100).
In summary, TILs are emerging as a valuable prognostic and potentially predictive biomarker in eTNBC, providing important information about the tumor’s immune landscape and response to therapy. Some international consensuses, such as European Society for Medical Oncology (ESMO) and St. Gallen (101,102), endorse the prognostic relevance of TILs and advocate the routine inclusion of TILs count in the pathological report. However, we recognize that the current data are mostly based on retrospective exploratory analyses and do not meet the criteria for clinical utility, defined by Hayes et al., which consider the analytical validity of the test, the significance of related results, and the magnitude of impact, in addition to the level of evidence that determines the applicability of the test (103). Further validation with prospective data are lacking to support the use of this biomarker to optimize and individualize our current therapeutic strategies for these patients.
Future perspectives
Biomarker development remains a very challenging endeavor. We clearly recognize different populations in clinical practice but lack effective tools to set apart groups of patients that may have different biology and require different therapeutic strategies. Translating knowledge of TILs into clinical practice and their use as an effective and reliable biomarker faces several difficulties related to tumor heterogeneity, dynamic variability of TILs, data interpretation, different assessment techniques and the complexity of the TME. In addition to the proposal of the TILs working group, new technologies, such as automated methods of immunofluorescence image analysis, next-generation sequencing (NGS) and the use of transcriptomic data, can also contribute to a greater understanding and precision in the quantification of TILs and potentially improve clinical applicability. Among other references, a transcriptomic signature was correlated with TILs assessed by histology in a cohort of patients with early BC. The declared signature was found to be a good biomarker associated with DFS and OS in an analysis adjusted for molecular and clinical variables, with better survival in basal and HER2 tumor types (104). The use of multiplexed immunofluorescent imaging and NGS that can determine the spatial distribution of specific immunophenotypes has also showed an impact on TNBC. A study evaluated the development and validation of a gene classifier for spatial immunophenotype and found positive results with response to checkpoint inhibitor (anti-PD1) treatment independently of currently used clinical markers (105). In the future, digital pathology, machine learning techniques, and better identification of TILs subpopulations will help to standardize pathology evaluation and expand our ability to validate tumor immune infiltration as a clinically meaningful biomarker (106).
Despite the growing amount of information on the subject, more clinical research and prospective translational studies are needed to unravel the potential role of TILs in guiding therapy choices. Several ongoing clinical trials are exploring the use of TILs and their potential impact on the treatment and outcomes of eTNBC. High TILs are being considered as an inclusion criterion for therapeutic personalization strategies. For example, the NeoTRACT study (NCT05645380) uses an early assessment combining radiologic response and TILs status to de-escalate chemotherapy (anthracycline-free protocol). This is a non-randomized phase II study that evaluates a treatment de-escalation strategy according to the expression of initial TILs (<5%, 5–29%, and ≥30%) whose primary objective is pCR rate in a high-sTIL cohort with radiographic complete response. The Dutch BELLINI (107) study demonstrates that a significant proportion of patients with TNBC and high TILs had marked immune activation after 4 weeks of neoadjuvant therapy with ICI, highlighting the potential for developing randomized clinical trials with immunotherapy without chemotherapy for selected patients in this setting.
On a different perspective, the outstanding achievements of chimeric antigen receptor (CAR)-T cell therapy in hematological malignancies and the promising effects of adaptive cell therapies (ACTs) in solid tumors have prompted the search for more suitable targets or combination programs to expand this therapeutic approach to solid tumors. ACT with expansion of TILs may more accurately identify targets in tumor cells (108). Strategies in development have been focused on metastatic disease, where case reports with favorable outcomes have already been described. However, it is possible to envisage applying strategies involving ACT in treating earlier stages of BC. Other forms of immunotherapy, such as vaccines and antibody-drug conjugates, are also being extensively studied in eTNBC. TILs may also be useful as biomarkers in these scenarios (109).
Conclusions
Even though we have advanced in our understanding of the disease, the increasingly and evolving complex biology of cancer remains a challenge and compromises our ability to improve outcomes. Both progression of the disease and resistance development represent formidable hurdles. As the current tumor genetic sequencing associated excitement seems to be wearing off, progressively, we are focusing on other important aspects of the disease and discovering host-related tumor enabling properties that are potential treatment targets. The study of TILs falls in this scenario. The expression of TILs in early TNBC has shown clinical relevance in several studies that are consistent in showing the association of higher density of TILs and favorable outcomes in terms of both survival and response to neoadjuvant treatment. This impact supports the development of studies that evaluate the expression of TILs as stratification factors or the conduct of studies that can evaluate the usefulness and clinical validation of the biomarker. While the mere presence of TILs seems rather consistently to have a prognostic impact, more granular characterization of the type of lymphocytes seems to be required to better understand the biological significance of the infiltrate and adds value to prognostication.
Although the therapeutic impact of the immune system has been clearly established in many different tumors, better characterization of the specific immune response in a given tumor will certainly better inform and qualify our therapeutic strategy. Imbedded in the very challenging endeavor of biomarker development, we need more well-conducted clinical studies to address both the prognostic and predictive value of TILs. The evidence we present, supports that the lymphocyte infiltration in early BC seems to have a prognostic impact that can be clinically useful to characterize patient populations with very different biology and that can be amenable to escalation or de-escalation strategies. Ongoing and future studies should shed light on the clinical practical application of the immune infiltration in early BC.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-43/coif). C.H.B. has received grants or contracts to the institution from Nektar, Pfizer, Polyphor, Amgen, Daiichi Sankyo, Sanofi, Exelixis, Regeneron, Novartis, GSK, Janssen, OBI Pharma, Lilly, Seagen, Roche, BMS, MSD, Merck Serono, AstraZeneca, Novocure, Aveo Oncology, Takeda, TRIO, PharmaMar, Celgene, PPD, Syneos Health, Labcorp, ICON, IQVIA, Parexel, Nuvisan, PSI, Worldwide, Gilead Sciences, Bayer, Servier; consulting fees from Boehringer-Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, Eisai, Bayer, MSD, AstraZeneca, Zodiac, Lilly, Sanofi, Daiichi; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Boehringer-Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, Eisai, Bayer, MSD, Astra Zeneca, Zodiac, Lilly, Sanofi, Daiichi; support for attending meetings and/or travel from Novartis, Pfizer, Roche/Genentech, MSD, Astra Zeneca, Lilly, Daiichi; participation on a Data Safety Monitoring Board or Advisory Board from Roche; stock from Thummi and MEDSIR. T.R. has received speaker’s honoraria from Daiichi-Sankyo, AstraZeneca, Gilead Libbs, Pfizer, MSD and Novartis; consulting fees for advisory boards from AstraZeneca, Lilly, MSD and Novartis; research funding from AstraZeneca, FAPESP, Libbs, PRONOM/MS. G.S. has received speaker’s honoraria from Daiichi-Sankyo, AstraZeneca, and Novartis; and consulting fees for advisory boards, and support for attending meetings from AstraZeneca. M.G. has received speaker’s honoraria from Daiichi-Sankyo, AstraZeneca and Novartis; consulting fees for advisory boards from AstraZeneca and Novartis; research funding from AstraZeneca and Libbs. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Lüönd F, Tiede S, Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer 2021;125:164-75. [Crossref] [PubMed]
- Luo R, Li Y, He M, et al. Distinct biodistribution of doxorubicin and the altered dispositions mediated by different liposomal formulations. Int J Pharm 2017;519:1-10. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Parise CA, Bauer KR, Brown MM, et al. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999-2004. Breast J 2009;15:593-602. [Crossref] [PubMed]
- Loizides S, Constantinidou A. Triple negative breast cancer: Immunogenicity, tumor microenvironment, and immunotherapy. Front Genet 2023;13:1095839. [Crossref] [PubMed]
- Oualla K, Kassem L, Nouiakh L, et al. Immunotherapeutic Approaches in Triple-Negative Breast Cancer: State of the Art and Future Perspectives. Int J Breast Cancer 2020;2020:8209173. [Crossref] [PubMed]
- García-Teijido P, Cabal ML, Fernández IP, et al. Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin Med Insights Oncol 2016;10:31-9. [Crossref] [PubMed]
- Mediratta K, El-Sahli S, D'Costa V, et al. Current Progresses and Challenges of Immunotherapy in Triple-Negative Breast Cancer. Cancers (Basel) 2020;12:3529. [Crossref] [PubMed]
- Bao C, Lu Y, Chen J, et al. Exploring specific prognostic biomarkers in triple-negative breast cancer. Cell Death Dis 2019;10:807. [Crossref] [PubMed]
- Kudelova E, Smolar M, Holubekova V, et al. Genetic Heterogeneity, Tumor Microenvironment and Immunotherapy in Triple-Negative Breast Cancer. Int J Mol Sci 2022;23:14937. [Crossref] [PubMed]
- Cocco S, Piezzo M, Calabrese A, et al. Biomarkers in Triple-Negative Breast Cancer: State-of-the-Art and Future Perspectives. Int J Mol Sci 2020;21:4579. [Crossref] [PubMed]
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [Crossref] [PubMed]
- Zagami P, Carey LA. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022;8:95. [Crossref] [PubMed]
- Bianchini G, De Angelis C, Licata L, et al. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol 2022;19:91-113. [Crossref] [PubMed]
- Gupta RK, Roy AM, Gupta A, et al. Systemic Therapy De-Escalation in Early-Stage Triple-Negative Breast Cancer: Dawn of a New Era? Cancers (Basel) 2022;14:1856. [Crossref] [PubMed]
- Heil J, Kuerer HM, Pfob A, et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: current evidence and future challenges. Ann Oncol 2020;31:61-71. [Crossref] [PubMed]
- Denduluri N, Somerfield MR, Eisen A, et al. Selection of Optimal Adjuvant Chemotherapy Regimens for Human Epidermal Growth Factor Receptor 2 (HER2) -Negative and Adjuvant Targeted Therapy for HER2-Positive Breast Cancers: An American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol 2016;34:2416-27. Erratum in: J Clin Oncol 2017;35:263 Erratum in: J Clin Oncol 2017;35:1140. [Crossref] [PubMed]
- Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol 2021;39:1485-505. [Crossref] [PubMed]
- Abuhadra N, Stecklein S, Sharma P, et al. Early-stage Triple-negative Breast Cancer: Time to Optimize Personalized Strategies. Oncologist 2022;27:30-9. [Crossref] [PubMed]
- von Minckwitz G, Martin M. Neoadjuvant treatments for triple-negative breast cancer (TNBC). Ann Oncol 2012;23:vi35-9. [Crossref] [PubMed]
- Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13-21. [Crossref] [PubMed]
- Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24:2019-27. [Crossref] [PubMed]
- Bian L, Yu P, Wen J, et al. Survival benefit of platinum-based regimen in early stage triple negative breast cancer: A meta-analysis of randomized controlled trials. NPJ Breast Cancer 2021;7:157. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396:1090-100. [Crossref] [PubMed]
- Winer EP, Lipatov O, Im SA, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:499-511. [Crossref] [PubMed]
- Jacobs F, Agostinetto E, Miggiano C, et al. Hope and Hype around Immunotherapy in Triple-Negative Breast Cancer. Cancers (Basel) 2023;15:2933. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Agostinetto E, Losurdo A, Nader-Marta G, et al. Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer. Expert Opin Investig Drugs 2022;31:567-91. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Santa-Maria CA, O'Donnell M, Nunes R, et al. Integrating Immunotherapy in Early-Stage Triple-Negative Breast Cancer: Practical Evidence-Based Considerations. J Natl Compr Canc Netw 2022;20:738-44. [Crossref] [PubMed]
- Santa-Maria CA. Optimizing and Refining Immunotherapy in Breast Cancer. JCO Oncol Pract 2023;19:190-1. [Crossref] [PubMed]
- Kuroi K, Toi M, Ohno S, et al. Prognostic significance of subtype and pathologic response in operable breast cancer; a pooled analysis of prospective neoadjuvant studies of JBCRG. Breast Cancer 2015;22:486-95. [Crossref] [PubMed]
- Downs-Canner S, Mittendorf EA. Preoperative Immunotherapy Combined with Chemotherapy for Triple-Negative Breast Cancer: Perspective on the KEYNOTE-522 Study. Ann Surg Oncol 2023;30:3166-9. [Crossref] [PubMed]
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- Hahnen E, Lederer B, Hauke J, et al. Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer: Secondary Analysis of the GeparSixto Randomized Clinical Trial. JAMA Oncol 2017;3:1378-85. [Crossref] [PubMed]
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384:2394-405. [Crossref] [PubMed]
- Soysal SD, Tzankov A, Muenst SE. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015;82:142-52. [Crossref] [PubMed]
- Terceiro LEL, Edechi CA, Ikeogu NM, et al. The Breast Tumor Microenvironment: A Key Player in Metastatic Spread. Cancers (Basel) 2021;13:4798. [Crossref] [PubMed]
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012;125:5591-6. [Crossref] [PubMed]
- Li JJ, Tsang JY, Tse GM. Tumor Microenvironment in Breast Cancer-Updates on Therapeutic Implications and Pathologic Assessment. Cancers (Basel) 2021;13:4233. [Crossref] [PubMed]
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018;32:1267-84. [Crossref] [PubMed]
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [Crossref] [PubMed]
- El Bairi K, Haynes HR, Blackley E, et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ Breast Cancer 2021;7:150. [Crossref] [PubMed]
- Fan Y, He S. The Characteristics of Tumor Microenvironment in Triple Negative Breast Cancer. Cancer Manag Res 2022;14:1-17. [Crossref] [PubMed]
- Zhou Y, Tian Q, Wang BY, et al. The prognostic significance of TILs as a biomarker in triple-negative breast cancer: what is the role of TILs in TME of TNBC? Eur Rev Med Pharmacol Sci 2021;25:2885-97. [PubMed]
- Ahn SG, Jeong J, Hong S, et al. Current Issues and Clinical Evidence in Tumor-Infiltrating Lymphocytes in Breast Cancer. J Pathol Transl Med 2015;49:355-63. [Crossref] [PubMed]
- Yazaki S, Shimoi T, Yoshida M, et al. Integrative prognostic analysis of tumor-infiltrating lymphocytes, CD8, CD20, programmed cell death-ligand 1, and tertiary lymphoid structures in patients with early-stage triple-negative breast cancer who did not receive adjuvant chemotherapy. Breast Cancer Res Treat 2023;197:287-97. [Crossref] [PubMed]
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949-55. [Crossref] [PubMed]
- Lee S, Cho EY, Park YH, et al. Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol 2013;52:73-81. [Crossref] [PubMed]
- Hendry S, Salgado R, Gevaert T, et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol 2017;24:311-35. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105-13. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40-50. [Crossref] [PubMed]
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959-66. [Crossref] [PubMed]
- Pruneri G, Gray KP, Vingiani A, et al. Tumor-infiltrating lymphocytes (TILs) are a powerful prognostic marker in patients with triple-negative breast cancer enrolled in the IBCSG phase III randomized clinical trial 22-00. Breast Cancer Res Treat 2016;158:323-31. [Crossref] [PubMed]
- Tian T, Ruan M, Yang W, et al. Evaluation of the prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers. Oncotarget 2016;7:44395-405. [Crossref] [PubMed]
- Gao ZH, Li CX, Liu M, et al. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta-analysis. BMC Cancer 2020;20:1150. [Crossref] [PubMed]
- Sukumar J, Gast K, Quiroga D, et al. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther 2021;21:135-48. [Crossref] [PubMed]
- Kimura Y, Sasada S, Emi A, et al. (18)F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Predicts Tumor Immune Microenvironment Function in Early Triple-negative Breast Cancer. Anticancer Res 2023;43:127-36. [Crossref] [PubMed]
- Agarwal G, Vishvak Chanthar KMM, Katiyar S, et al. Predictive and Prognostic Role of Tumor-Infiltrating Lymphocytes in Patients with Advanced Breast Cancer Treated with Primary Systemic Therapy. World J Surg 2023;47:1238-46. [Crossref] [PubMed]
- Candelaria RP, Spak DA, Rauch GM, et al. BI-RADS Ultrasound Lexicon Descriptors and Stromal Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. Acad Radiol 2022;29:S35-41. [Crossref] [PubMed]
- de Jong VMT, Wang Y, Ter Hoeve ND, et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J Clin Oncol 2022;40:2361-74. [Crossref] [PubMed]
- Stecklein SR, Yoder R, Staley JM, et al. Differential impact of proliferation signature on efficacy of neoadjuvant chemoimmunotherapy in sTIL-high and sTIL-low triple-negative breast cancer (TNBC): Biomarker analysis of the NeoPACT trial. J Clin Oncol 2023;41:abstr 507.
- Sharma P, Stecklein SR, Yoder R, et al. Clinical and biomarker results of neoadjuvant phase II study of pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer (TNBC)(NeoPACT). J Clin Oncol 2022;40:abstr 513.
- Gluz O, Nitz U, Kolberg-Liedtke C, et al. De-escalated Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer (TNBC): Impact of Molecular Markers and Final Survival Analysis of the WSG-ADAPT-TN Trial. Clin Cancer Res 2022;28:4995-5003. [Crossref] [PubMed]
- Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279-88. [Crossref] [PubMed]
- Galvez M, Castaneda CA, Sanchez J, et al. Clinicopathological predictors of long-term benefit in breast cancer treated with neoadjuvant chemotherapy. World J Clin Oncol 2018;9:33-41. [Crossref] [PubMed]
- O'Loughlin M, Andreu X, Bianchi S, et al. Reproducibility and predictive value of scoring stromal tumour infiltrating lymphocytes in triple-negative breast cancer: a multi-institutional study. Breast Cancer Res Treat 2018;171:1-9. [Crossref] [PubMed]
- Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol 2016;27:249-56. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983-91. [Crossref] [PubMed]
- Wang K, Xu J, Zhang T, et al. Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: A meta-analysis. Oncotarget 2016;7:44288-98. [Crossref] [PubMed]
- Ovcaricek T, Matos E, Auprih M, et al. Prognostic value of systemic inflammatory response in early-stage triple-negative breast cancer. J Clin Oncol 2023;41:abstr e14562.
- Zhu Y, Tzoras E, Matikas A, et al. Expression patterns and prognostic implications of tumor-infiltrating lymphocytes dynamics in early breast cancer patients receiving neoadjuvant therapy: A systematic review and meta-analysis. Front Oncol 2022;12:999843. [Crossref] [PubMed]
- Bianchini G, Huang CS, Egle D, et al. LBA13 Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann Oncol 2020;31:S1145-6. [Crossref]
- Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014;25:611-8. [Crossref] [PubMed]
- De Jong VMT, Wang Y, Opdam M, et al. 159O Prognostic value of tumour infiltrating lymphocytes in young triple negative breast cancer patients who did not receive adjuvant systemic treatment; by the PARADIGM study group. Ann Oncol 2020;31:S303. [Crossref]
- Loi S, Salgado R, Adams S, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer 2022;8:3. [Crossref] [PubMed]
- Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol 2019;30:1941-9. [Crossref] [PubMed]
- Leon-Ferre RA, Polley MY, Liu H, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat 2018;167:89-99. [Crossref] [PubMed]
- Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol 2015;26:1698-704. [Crossref] [PubMed]
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860-7. [Crossref] [PubMed]
- Bonadio RC, Tarantino P, Testa L, et al. Management of patients with early-stage triple-negative breast cancer following pembrolizumab-based neoadjuvant therapy: What are the evidences? Cancer Treat Rev 2022;110:102459. [Crossref] [PubMed]
- Liu F, Lang R, Zhao J, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 2011;130:645-55. [Crossref] [PubMed]
- Gu-Trantien C, Loi S, Garaud S, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013;123:2873-92. [Crossref] [PubMed]
- Kim S, Lee A, Lim W, et al. Zonal difference and prognostic significance of foxp3 regulatory T cell infiltration in breast cancer. J Breast Cancer 2014;17:8-17. [Crossref] [PubMed]
- Ravelli A, Roviello G, Cretella D, et al. Tumor-infiltrating lymphocytes and breast cancer: Beyond the prognostic and predictive utility. Tumour Biol 2017;39:1010428317695023. [Crossref] [PubMed]
- Liu S, Foulkes WD, Leung S, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 2014;16:432. [Crossref] [PubMed]
- Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006;24:5373-80. [Crossref] [PubMed]
- Abdullaeva S, Semiglazova T, Artemyeva A, et al. Tumor-infiltrating lymphocytes (TILs) for prediction of response to platinum-based neoadjuvant chemotherapy (NACT) in triple-negative breast cancer (TNBC). J Clin Oncol 2023;41:abstr e12620.
- Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol 2020;31:569-81. [Crossref] [PubMed]
- Herrero-Vicent C, Guerrero A, Gavilá J, et al. Predictive and prognostic impact of tumour-infiltrating lymphocytes in triple-negative breast cancer treated with neoadjuvant chemotherapy. Ecancermedicalscience 2017;11:759. [Crossref] [PubMed]
- Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer 2018;25:34-42. [Crossref] [PubMed]
- Hida AI, Sagara Y, Yotsumoto D, et al. Prognostic and predictive impacts of tumor-infiltrating lymphocytes differ between Triple-negative and HER2-positive breast cancers treated with standard systemic therapies. Breast Cancer Res Treat 2016;158:1-9. [Crossref] [PubMed]
- Rao N, Qiu J, Wu J, et al. Significance of Tumor-Infiltrating Lymphocytes and the Expression of Topoisomerase IIα in the Prediction of the Clinical Outcome of Patients with Triple-Negative Breast Cancer after Taxane-Anthracycline-Based Neoadjuvant Chemotherapy. Chemotherapy 2017;62:246-55. [Crossref] [PubMed]
- Valenza C, Taurelli Salimbeni B, Santoro C, et al. Tumor Infiltrating Lymphocytes across Breast Cancer Subtypes: Current Issues for Biomarker Assessment. Cancers (Basel) 2023;15:767. [Crossref] [PubMed]
- Campbell MJ, Yau C, Bolen J, et al. Abstract CT003: Analysis of immune cell infiltrates as predictors of response to the checkpoint inhibitor pembrolizumab in the neoadjuvant I-SPY 2 TRIAL. Cancer Res 2019;79:CT003. [Crossref]
- Erber R, Kolberg HC, Schumacher J, et al. Association between pCR, TILs, and Ki-67 at baseline and after 2 weeks in patients with triple-negative breast cancer (TNBC) treated with atezolizumab and chemotherapy+/-a preceding atezolizumab monotherapy window: A translational analysis of the neoMono trial. J Clin Oncol 2023;41:abstr 595.
- Loibl S, Schneeweiss A, Huober JB, et al. Durvalumab improves long-term outcome in TNBC: results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J Clin Oncol 2021;39:abstr 506.
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49. [Crossref] [PubMed]
- Burstein HJ, Curigliano G, Thürlimann B, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 2021;32:1216-35. [Crossref] [PubMed]
- Hayes DF. Defining Clinical Utility of Tumor Biomarker Tests: A Clinician's Viewpoint. J Clin Oncol 2021;39:238-48. [Crossref] [PubMed]
- Truntzer C, Isambert N, Arnould L, et al. Prognostic value of transcriptomic determination of tumour-infiltrating lymphocytes in localised breast cancer. Eur J Cancer 2019;120:97-106. [Crossref] [PubMed]
- Hammerl D, Martens JWM, Timmermans M, et al. Spatial immunophenotypes predict response to anti-PD1 treatment and capture distinct paths of T cell evasion in triple negative breast cancer. Nat Commun 2021;12:5668. [Crossref] [PubMed]
- Loi S, Michiels S, Adams S, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol 2021;32:1236-44. [Crossref] [PubMed]
- Nederlof I, Isaeva OI, Bakker N, et al. LBA13 Nivolumab and ipilimumab in early-stage triple negative breast cancer (TNBC) with tumor-infiltrating lymphocytes (TILs): First results from the BELLINI trial. Ann Oncol 2022;33:S1382. [Crossref]
- Li R, Cao L. The role of tumor-infiltrating lymphocytes in triple-negative breast cancer and the research progress of adoptive cell therapy. Front Immunol 2023;14:1194020. [Crossref] [PubMed]
- Zacharakis N, Huq LM, Seitter SJ, et al. Breast Cancers Are Immunogenic: Immunologic Analyses and a Phase II Pilot Clinical Trial Using Mutation-Reactive Autologous Lymphocytes. J Clin Oncol 2022;40:1741-54. [Crossref] [PubMed]
Cite this article as: Rosa ML, Reinert T, Pauletto MM, Sartori G, Graudenz M, Barrios CH. Implications of tumor-infiltrating lymphocytes in early-stage triple-negative breast cancer: clinical oncologist perspectives. Transl Breast Cancer Res 2024;5:4.


