
Individualize management for advanced breast cancer with pyrotinib-based anti-HER2 therapy: a case report
Highlight box
Key findings
• Pyrotinib-based anti-HER2 therapies achieved promising outcomes in a HER2-positive breast cancer patient with brain metastases.
What is known and what is new?
• The efficacy of pyrotinib-based therapy for HER2-positive advanced breast cancer is known, but this case contributes valuable insights to the potential of a combination of large and small molecules containing pyrotinib in the first and second-line treatment in HER2-positive advanced breast cancer. Furthermore, the use of NGS and MRD detection in guiding therapeutic decisions represents a precision medicine approach that was effectively implemented in this case.
What is the implication, and what should change now?
• The successful management of this case underscores the promising role of pyrotinib-based therapy in advanced HER2-positive breast cancer. It also highlights the importance of precision medicine in guiding treatment decisions. Moreover, NGS and MRD might be increasingly utilized for personalized treatment planning.
Introduction
Human epidermal growth factor receptor-2 (HER2)-positive breast cancer, accounting for 15–20% of all breast cancer, was characterized by an aggressive phenotype, elevated recurrence rates, and poor survival outcomes prior to the emergence of anti-HER2 therapy (1). The advent of anti-HER2 targeted drugs have yielded assuring anticancer activity in HER2-positive breast cancer. Such therapeutic agents encompass anti-HER2 antibodies and their derivatives, antibody-drug conjugate (ADC), and tyrosine kinase inhibitors (TKIs) (2-4).
Pyrotinib, an irreversible dual pan-ErbB receptor TKI, was first approved in 2018 for the treatment of HER2-positive advanced breast cancer (5). The data from PHOEBE study, a phase 3 trial, confirmed the efficacy and safety of pyrotinib in combination with capecitabine as second-line therapy (6). The ongoing PHILA study, another phase 3 clinical trial (NCT03863223), aims to evaluate efficacy and safety of pyrotinib plus trastuzumab and docetaxel versus placebo plus trastuzumab and docetaxel as a first-line therapy for HER2-positive metastatic breast cancer (7). Based on the findings of this study, the 2023 Chinese Society of Clinical Oncology (CSCO) guidelines endorsed trastuzumab and docetaxel plus pyrotinib as a Class 1 recommendation. Here, we report a case of metastatic HER2-positive breast cancer with multiple metastases, which yielded a noteworthy clinical benefit following treatment with pyrotinib. We present this article in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-26/rc).
Case presentation
A 33-year-old, female patient was diagnosed with hormone receptor (HR) positive, HER2-positive breast cancer patient and subsequently underwent left breast conserving surgery along with sentinel lymph node biopsy in June 2018. The postoperative pathology revealed a tumor size of 2.5×2.4×2.3 cm3, with immunohistochemistry (IHC) indicating HER2 (3+), estrogen receptor (ER, +, 3%), progesterone receptor (−), Ki-67 (+, 45%). The pathological stage was pT2N0M0 IIA. No adjuvant chemoradiotherapy or anti-HER2 targeted therapy was administered post-surgery. In May 2020, she presented with recurrent left breast mass accompanied by liver, bone, and multiple lymph node metastases and the disease-free survival (DFS) was 22.8 months. Grade 3 anemia at that time prevented the patient from undergoing chemotherapy, thus she was given the targeted therapy with pyrotinib 400 mg daily instead. Remarkably, the patient’s left breast tumor, lymph nodes, and liver lesions all dramatically decreased within less than one treatment cycle (16 days) of with oral pyrotinib therapy (Figure 1), evaluated as partial response (PR) as per the RECIST v1.1. Upon improvement of the patient’s physical condition, the combination therapy was introduced in second cycle, comprising of nab-paclitaxel (200 mg d1, 11), trastuzumab (8 mg/kg d1), and pyrotinib (400 mg qd) every three weeks until the thirteenth cycle when the therapy was adjusted to nab-paclitaxel (200 mg d1, 8), trastuzumab (6 mg/kg d1), and pyrotinib (400 mg qd) per three weeks. Concurrently, pamidronic acid was administered for bone metastases. Subsequently, depending on the patient’s condition and preferences, the maintenance therapy was altered to a double-targeted regimen, including trastuzumab (6 mg/kg d1) and pyrotinib (400 mg qd). The patient achieved PR after two cycles and the progression-free survival (PFS) was 16 months. In September 2021, brain metastases were detected accompanied by nausea, headaches, and dizziness, whereas extracranial lesions remained stable. Following craniocerebral palliative radiation, the patient further received regular vinorelbine (20 mg capsule or 40 mg injection d1, 8), inetetamab (6 mg/kg, first dose 8 mg/kg, d1) and pyrotinib (400 mg qd) for 3 weeks per cycle and achieved PR (Figure 2). The drug used to treat bone metastases was switched to denosumab. Extracranial lesions were also in a stable status. After 8 cycles, intracranial lesions progressed with the PFS of 7.1 months. Given the progression of the brain metastases, trastuzumab deruxtecan (300 mg) was given every three weeks. The patient’s brain metastases noticeably decreased after two cycles of treatment and attained PR with magnetic resonance imaging (MRI) evaluation indicating no significant mass (Figure 3). This patient has been undergoing treatment with trastuzumab deruxtecan, with the PFS extending beyond 12 months to date. No drug-related adverse events occurred throughout the treatment. The entire treatment timeline for this patient is presented in Figure 4.
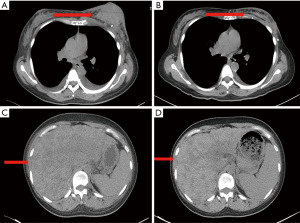
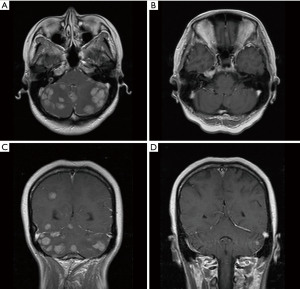
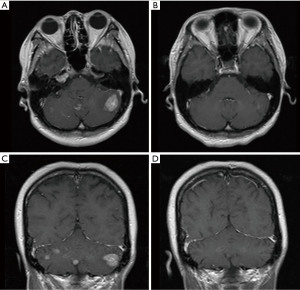

We employed the next generation sequencing (NGS) to identify the expression of 1,021 tumor-related genes from the surgical tissue. There were 24 somatic mutations, including 10 copy number variant amplifications, 11 copy number variant deletions and 3-point mutations (Table 1). The amplification of HER2 (ERBB2) revealed that the patient could potentially derive clinical benefit from anti-HER2 therapy, for instance, trastuzumab, pyrotinib, and trastuzumab deruxtecan. At present, the patient presents no measurable target lesions, thus, we utilized the plasma of the patient to test for minimal residual disease (MRD), which yielded a negative result. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Table 1
| Gene | Transcript | Mutation | Function zones | Variation frequency |
|---|---|---|---|---|
| TP53 | NM_000546.5 | c.833C>G: p.P278R | EX8 | 49.9% |
| CDK13 | NM_003718.4 | c.599G>C: p.R200P | EX1 | 45.6% |
| SPEN | NM_015001.2 | c.7135G>A: p.E2379K | EX11 | 23.1% |
| ERBB2 | NM_004448.2 | Amplification | All exon | 13.1 |
| CDK12 | NM_016507.2 | Amplification | All exon | 12.7 |
| ZNF703 | NM_025069.1 | Amplification | All exon | 6.0 |
| RAD21 | NM_006265.2 | Amplification | All exon | 2.3 |
| RPS20 | NM_001146227.1 | Amplification | All exon | 2.3 |
| WHSC1L1 | NM_023034.1 | Amplification | All exon | 2.2 |
| LYN | NM_002350.3 | Amplification | All exon | 2.1 |
| RSPO2 | NM_178565.4 | Amplification | All exon | 2.0 |
| FGFR1 | NM_023110.2 | Amplification | All exon | 2.0 |
| BRD4 | NM_058243.2 | Amplification | All exon | 2.0 |
| PTCH2 | NM_001166292.1 | Deletion | All exon | 0.7 |
| BRCA1 | NM_007294.3 | Deletion | All exon | 0.7 |
| ARID1A | NM_006015.4 | Deletion | All exon | 0.7 |
| STK11 | NM_000455.4 | Deletion | All exon | 0.7 |
| NF1 | NM_000267.3 | Deletion | All exon | 0.7 |
| ATM | NM_000051.3 | Deletion | All exon | 0.7 |
| MRE11A | NM_005591.3 | Deletion | All exon | 0.6 |
| CHEK1 | NM_001114121.2 | Deletion | All exon | 0.6 |
| ATR | NM_001184.3 | Deletion | All exon | 0.6 |
| RB1 | NM_000321.2 | Deletion | All exon | 0.5 |
| WRN | NM_000553.4 | Deletion | All exon | 0.5 |
Discussion
In this case, the patient achieved encouraging responses across multiple treatment regimens. The patient underwent breast conserving surgery with postoperative pathology indicating HER2-overexpression. Regrettably, patient compliance was subpar, resulting in the omission of radiation therapy and any anti-HER2 adjuvant targeted therapy. Approximately two years post-surgery, the patient developed metastases in liver, bone, and lymph nodes—a timeframe corresponding to the high-risk period for HER2-positive breast cancer (8). According to the ALTTO study, patients with HR-positive and HER2-positive breast cancer who received chemotherapy combined with anti-HER2-targeted therapy demonstrated a 4-year DFS rate of 85–92% (9). Only 8% to 15% of patients relapsed within 4 years, underscoring the indispensable role of adjuvant therapy. It’s critical that clinicians invest in communication with patients and involve them in the clinical decision-making process, particularly when managing breast cancer patients with suboptimal compliance. In these cases, clinicians should select appropriate anti-tumor drugs to foster patient confidence during the treatment process. We tried pyrotinib alone at the beginning, which resulted in remarkable efficacy within less than one treatment cycle. The mass of left breast visually decreased and the patient achieved PR, bolstering the patient’s confidence. In the second cycle, trastuzumab and nab-paclitaxel were added, obtaining a PFS of 16 months. In the PHILA study, patients who had not received trastuzumab, had not undergone neoadjuvant or adjuvant therapy, and developed liver metastases, all benefited from the regimen of pyrotinib plus trastuzumab and docetaxel. The objective response rate (ORR) was 82.8% (95% CI: 78.1–86.9%) and the median PFS (mPFS) was 24.3 months (95% CI: 19.1–33.0) in the pyrotinib group, compared to the ORR of 70.6% (95% CI: 65.1–75.8%) and mPFS of 10.4 months (95% CI: 9.3–12.3) in the placebo group (7). Despite substantial tumor burden, poor compliance, and the relatively young age in this case, the patient sustained a PFS of 16 months, affirming the encouraging efficacy of pyrotinib as the first-line therapy as corroborated by the of PHILA study.
Approximately 20–30% HER2-positive breast cancer patients harbor brain metastases, due to a biological predisposition for cerebral dissemination (10,11). The median survival time among the HER2-positive breast cancer with brain metastases was reported to be 10 months (12). The blood-brain barrier (BBB) typically hinders drug penetration into central nervous system, whereas, brain metastasis can compromise the integrity of BBB (13), allowing targeted agents can be absorbed by the intracranial metastases. Pyrotinib, a small-molecule TKI, can exceed the BBB and exhibits antitumor activity. Although there were several clinical studies supporting the effectiveness of pyrotinib, the data on its combination with monoclonal antibodies in the treatment of breast cancer with brain metastases were still sparse. Several previous studies have reported that trastuzumab and some TKI levels, such as tucatinib, lapatinib, and neratinib, could be detected in cerebrospinal fluid (14,15). Lin et al. enrolled 31 metastatic breast cancer patients with brain metastases to receive pyrotinib-based therapy, achieving a mPFS of 6.7 months and an intracranial ORR of 28% (16). Yan et al. reported on a trial of pyrotinib combined with capecitabine for the treatment of breast cancer with brain metastases. The phase 2 PERMEATE trial enrolled 78 HER2-positive breast cancer patients with brain metastases to assess the anti-tumor activity and safety of pyrotinib plus capecitabine and divided them into two cohorts, including radiotherapy-naïve (cohort A) and progressive disease post-radiotherapy (cohort B). The mPFS was 11.3 months (95% CI: 7.7–14.6) in cohort A and 5.6 months (95% CI: 3.4–10.0) in cohort B. The intracranial ORR was 74.6% and the extracranial ORR was 70.4% in cohort A (17). Therefore, metastatic HER2-positive breast cancer patients could potentially benefit from pyrotinib plus capecitabine in terms of survival, regardless of the presence or absence of brain metastases. Although this patient developed brain metastases, the extracranial lesions remained stable. Thus, following localized craniocerebral irradiation, we decided to continue pyrotinib treatment in compliance with 2023 CSCO guidelines. This patient received pyrotinib plus inetetamab and vinorelbine as the second-line treatment. Inetetamab, an analog of trastuzumab, combined with vinorelbine significantly prolonged PFS compared with vinorelbine alone in patients with HER2 positive metastatic breast cancer (39.1 vs. 14.0 weeks) in the HOPES study (18). Deng et al. systematically investigated the therapeutic regimen of inetetamab in combination with small molecules TKI, including pyrotinib, and concluded it had synergistic antitumor effects (19). Similar research has examined potential therapies in breast cancer with brain metastases for combination of TKI and monoclonal antibody. In the HER2CLIMB Trial, Lin et al. enroll 291 HER2-positive breast cancer patients with brain metastases to explore the intracranial efficacy and survival outcomes of tucatinib plus trastuzumab and capecitabine. The mPFS of this combination was 9.9 months (95% CI: 8.0–13.9), a marked improvement compared to 4.2 months (95% CI: 3.6–5.7) observed in the control group receiving placebo plus trastuzumab and capecitabine (20). This case contributes valuable insights to the existing limited data on the treatment of brain metastases with a combination of large and small molecules containing pyrotinib.
At present, several TKI have been used in the field of breast cancer, such as pyrotinib, tucatinib, lapatinib, neratinib, afatinib, etc. (21). As mentioned above, pyrotinib and tucatinib had demonstrated excellent clinical benefit in breast cancer patients with brain metastases. The clinical efficacy of pyrotinib and lapatinib was compared in the PHOEBE trial (6). A total of 267 patients with HER2-positive advanced breast cancer randomized to pyrotinib plus capecitabine group and lapatinib plus capecitabine group were included in the PHOEBE trial, and the results showed a clear benefit of mPFS in pyrotinib group (12.5 vs. 6.8 months, hazard ratio =0.39; 95% CI: 0.27–0.56). The ORR was 67.2% in pyrotinib group compared to 51.5% in lapatinib group and the safety of pyrotinib plus capecitabine was controllable. Pyrotinib plus capecitabine can be considered an alternative treatment option for patients with HER2-positive metastatic breast cancer. A meta-analysis, for a total of 799 HER2-positive advanced breast cancer patients with brain metastases, discussed the efficacy of lapatinib and assessed the ORR was 21.4% (22).
Trastuzumab deruxtecan (T-DXd; DS-8201), an ADC, was approved in December 2019 by Food and Drug Administration (FDA) for patients with unresectable or metastatic HER2-positive breast cancer who have been treated with at least two prior anti-HER2-based therapies (23). Data from the DESTINY-Breast01 subgroup, both with or without brain metastases, showed that the ORR was 58.3% and the mPFS was 18.1 months among patients with brain metastases (24). In the Destiny-Breast02 trial, mPFS reached 13.9 months in patients with brain metastases at baseline receiving trastuzumab deruxtecan (hazard ratio =0.35; 95% CI: 0.20–0.61) (25). Patients with brain metastases were also enrolled in the phase 3 Destiny-Breast03 trial, and the mPFS was 15.0 month for trastuzumab deruxtecan, compared to 5.7 months for TDM1 (hazard ratio =0.38; 95% CI: 0.23–0.64) (26). Consequently, trastuzumab deruxtecan seems to have promising potential to treat advanced breast cancer patients with brain metastases after pyrotinib-based therapy. The patient in this case underwent pyrotinib-based therapy for approximately 23 months and had the chance to utilize another sensitive drug due to our collaborative clinical decision-making approach tailored to her individual circumstances. Additionally, the patient in this case obtained an OS over 35 months, demonstrating the significant survival effects of anti-HER2 drugs exemplified by pyrotinib.
Under the guidance of precision medicine, clinicians ought to utilize novel diagnostic and treatment approaches to facilitate patients in crafting individualized clinical strategies. For this patient, we performed the NGS and MRD testing. The patient had BRCA1 and ATM mutations, known as breast cancer susceptibility genes. Patients carrying germline BRCA1 mutation exhibit a higher propensity for developing brain metastases than those without mutations (27). In this case, the patient carrying somatic BRCA1 mutation may also be associated with poor prognosis, necessitating early genetic counseling or intensive screening to mitigate the risk of breast cancer recurrence. The patient carried mutations in TP53, a gene typically characterized by a low mutation rate in HR-positive, HER2-positive breast cancer (28), which could potentially affect the treatment efficacy. Nevertheless, pyrotinib-based therapy showed superiority in both first and second-line treatments. The burden of MRD is related to the risk of recurrence (29). Current studies suggest a better prognosis for patients who test negative for MRD in peripheral blood (30). However, for the patient with brain metastases in this case, it could not be ruled out that the obstruction of the BBB may impede the effectiveness of MRD testing. Hence, it might be beneficial to conduct a MRD testing prior to the initiation of treatment, which could serve as a baseline reference for subsequent evaluation of therapeutic efficacy. For the patients with complete response, further MRD testing would provide guidance for determining whether to maintain the current treatment or modify the anti-HER2 therapy. For instance, some patients may test negative in their baseline MRD testing and show signs of improvement in their imaging after treatment, but if their MRD testing comes back positive, these patients should be considered as experiencing a recurrence and their treatment strategy should be altered. Therefore, continuous monitoring is vital during follow-up therapy to facilitate the timely provision of optimized and personalized treatment plans.
We acknowledge that this case has some limitations. Upon the patient’s detection of brain metastases, she had obvious clinical symptoms and cranial MRI showed multiple lesions. Consequently, it is advisable for clinicians managing patients with advanced breast cancer to routinely review cranial MRI, even when the patient appears satisfactory results. Furthermore, clinicians can enhance the management of HER2-positive breast cancer patients more effectively by integrating the findings from precision medicine technologies such as NGS and MRD into their treatment strategies.
Conclusions
In summary, we presented a typical case that pyrotinib as a promising antineoplastic agent for HER2-positive advanced breast cancer patients. The utilization of novel diagnostic and therapeutic methods to manage advanced breast cancer patients is in line with the concept of precision medicine. We believe that such an effort will inspire clinicians to pursue more effective and precise treatments.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-26/rc
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-26/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-26/coif). XW serves as an unpaid editorial board member of Translational Breast Cancer Research from March 2022 to February 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Choong GM, Cullen GD, O'Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J Clin 2020;70:355-74. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Rassy E, Delaloge S. A second-generation antibody-drug conjugate to treat HER2-positive breast cancer. Lancet 2023;401:80-1. [Crossref] [PubMed]
- Chien AJ, Rugo HS. Tyrosine Kinase Inhibitors for Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer: Is Personalizing Therapy Within Reach? J Clin Oncol 2017;35:3089-91. [Crossref] [PubMed]
- Blair HA. Pyrotinib: First Global Approval. Drugs 2018;78:1751-5. [Crossref] [PubMed]
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Xu B, Yan M, Ma F, et al. Pyrotinib or placebo in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer (PHILA): a randomized phase III trial. Ann Oncol 2022;33:abstr LBA19.
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [Crossref] [PubMed]
- Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol 2016;34:1034-42. [Crossref] [PubMed]
- Hurvitz SA, O'Shaughnessy J, Mason G, et al. Central Nervous System Metastasis in Patients with HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from SystHERs. Clin Cancer Res 2019;25:2433-41. [Crossref] [PubMed]
- Venur VA, Leone JP. Targeted Therapies for Brain Metastases from Breast Cancer. Int J Mol Sci 2016;17:1543. [Crossref] [PubMed]
- Martin AM, Cagney DN, Catalano PJ, et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol 2017;3:1069-77. [Crossref] [PubMed]
- Watase C, Shiino S, Shimoi T, et al. Breast Cancer Brain Metastasis-Overview of Disease State, Treatment Options and Future Perspectives. Cancers (Basel) 2021;13:1078. [Crossref] [PubMed]
- Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 2007;18:23-8. [Crossref] [PubMed]
- Li J, Jiang J, Bao X, et al. Mechanistic Modeling of Central Nervous System Pharmacokinetics and Target Engagement of HER2 Tyrosine Kinase Inhibitors to Inform Treatment of Breast Cancer Brain Metastases. Clin Cancer Res 2022;28:3329-41. [Crossref] [PubMed]
- Lin Y, Lin M, Zhang J, et al. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res Treat 2020;52:1059-66. [Crossref] [PubMed]
- Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol 2022;23:353-61. [Crossref] [PubMed]
- Bian L, Xu BH, Di LJ, et al. Phase III randomized controlled, multicenter, prospective study of recombinant anti-HER2 humanized monoclonal antibody (Cipterbin) combined with vinorelbine in patients with HER2 positive metastatic breast cancer: the HOPES Study. Zhonghua Yi Xue Za Zhi 2020;100:2351-7. [Crossref] [PubMed]
- Deng L, Zhao L, Liu L, et al. Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers. Open Life Sci 2023;18:20220535. [Crossref] [PubMed]
- Lin NU, Borges V, Anders C, et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol 2020;38:2610-9. [Crossref] [PubMed]
- Le Du F, Diéras V, Curigliano G. The role of tyrosine kinase inhibitors in the treatment of HER2+ metastatic breast cancer. Eur J Cancer 2021;154:175-89. [Crossref] [PubMed]
- Petrelli F, Ghidini M, Lonati V, et al. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur J Cancer 2017;84:141-8. [Crossref] [PubMed]
- Narayan P, Osgood CL, Singh H, et al. FDA Approval Summary: Fam-Trastuzumab Deruxtecan-Nxki for the Treatment of Unresectable or Metastatic HER2-Positive Breast Cancer. Clin Cancer Res 2021;27:4478-85. [Crossref] [PubMed]
- Jerusalem G, Park YH, Yamashita T, et al. Trastuzumab Deruxtecan in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A DESTINY-Breast01 Subgroup Analysis. Cancer Discov 2022;12:2754-62. [Crossref] [PubMed]
- André F, Hee Park Y, Kim SB, et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 2023;401:1773-85. [Crossref] [PubMed]
- Cortés J, Kim SB, Chung WP, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 2022;386:1143-54. [Crossref] [PubMed]
- Fasching PA, Yadav S, Hu C, et al. Mutations in BRCA1/2 and Other Panel Genes in Patients With Metastatic Breast Cancer -Association With Patient and Disease Characteristics and Effect on Prognosis. J Clin Oncol 2021;39:1619-30. [Crossref] [PubMed]
- Brandão M, Caparica R, Malorni L, et al. What Is the Real Impact of Estrogen Receptor Status on the Prognosis and Treatment of HER2-Positive Early Breast Cancer? Clin Cancer Res 2020;26:2783-8. [Crossref] [PubMed]
- Coakley M, Garcia-Murillas I, Turner NC. Molecular Residual Disease and Adjuvant Trial Design in Solid Tumors. Clin Cancer Res 2019;25:6026-34. [Crossref] [PubMed]
- Garcia-Murillas I, Chopra N, Comino-Méndez I, et al. Assessment of Molecular Relapse Detection in Early-Stage Breast Cancer. JAMA Oncol 2019;5:1473-8. [Crossref] [PubMed]
Cite this article as: Xu J, Chen J, Huang P, Zhou H, Wang X, Chen Z. Individualize management for advanced breast cancer with pyrotinib-based anti-HER2 therapy: a case report. Transl Breast Cancer Res 2023;4:25.


