Self-detection remains a primary means of breast cancer detection in Beijing, China
Introduction
Breast cancer is the most common malignancy in women worldwide, causing an increasing socioeconomic burden. Despite a lower but increasing incidence, breast cancer in China is more likely to be identified at advanced stages compared to western counties (1), and relevantly the 5-year survival rate is lower (2). The needs of improving early detection and early treatment of breast cancer are immediate and substantial.
Traditional methods of breast cancer detection include breast self-examination (BSE) and clinical breast examination (CBE), by which means tumors are only detectable after symptoms and/or signs onset. With the advent of the mammographic screening, detection of subclinical diseases became possible. Benefit on long-term survival from organized (annual or biennial) mammographic screening in asymptomatic populations has been proven in randomized controlled trials and is widely accepted based on experiences from developed countries (3,4). However, national-wide mammographic screening program is impractical in many low- and middle-income countries. Instead, CBE by experienced physicians has been recommended as both an educational and screening tool in resource-limited areas (5).
In China, screening starts relatively late at the dawn of the 21st century. And despite implementation of several large-scale screening programs such as the 2008 Chinese National Breast Cancer Screening Program, only a small fraction of the entire population was covered (6). A cross-sectional study conducted in Beijing several years ago involving more than 3,000 individuals diagnosed with primary breast cancer in 2008 reported a screen-detection rate of only 5.2%, which was much lower compared to the USA over the same period (about 58%) (7,8).
This study aimed to reveal the current situation of breast cancer detection and screening in Beijing over a decade of efforts, and to determine potential barriers to screening in a Chinese breast cancer patient cohort. We believe such knowledge may guide in designing more rational and effective preventive strategies in the future. We present this article in accordance with the STROBE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-2/rc).
Methods
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Ethics Committee of Tsinghua Changgung Hospital (No. 21381-0-01). Informed consent was obtained from all individual participants included in the study.
Study population
All female patients who were newly diagnosed with histologically confirmed breast cancer in Beijing Tsinghua Changgung Hospital from Jan 2015 to July 2021 were eligible for this study. After excluding 13 deceased patients, 310 of 344 (90.1%) consecutive patients who were living at the time of the study consented to participate. Eight patients refused to participate and the remaining 26 were lost follow-up.
Data collection and variable definitions
Electronic medical records were retrieved including age, body mass index (BMI), clinical notes, radiology images and reports, operation records and pathological reports. Required information on screening history and socioeconomic status were obtained through face-to-face or phone interviews by trained interviewers using a structured questionnaire, including mode of detection, screening frequency, screening modality, self-perceived barrier to screening, education level, type of insurance, family income and working status. Disease stage at diagnosis was assigned according to 2017 American Joint Committee on Cancer (AJCC) Cancer Staging Manual (8th edition). Patients were categorized as ever-screened (ES) or never-screened (NS) group based on their past screening behavior.
Statistical analysis
Statistical analysis was performed using IBM SPSS software, version 25.0, and Graphpad Prism software, version 8.0. Differences in categorial variables such as marital status between two groups were compared using chi-square tests or Fisher’s exact tests as appropriate. Differences in continuous variables such as age and BMI were compared with t-tests. All tests were two-sided, and a P value of <0.05 was considered statistically significant.
Results
Mode of detection and cancer stage
The mean age at diagnosis of our study cohort was 57.5 years, with a range of 27 to 90 years. Mode of detection and initial breast symptoms and signs were summarized in Table 1. A total of 225 (72.6%) had self-detected diseases, 95.1% through a palpable breast lump. Less commonly seen symptoms and signs included nipple discharge, change of breast size, dimple sign, peau d’orange, nipple retraction, etc., each accounting for less than 10%. A total of 77 (24.8%) were detected by screening, mostly by ultrasonography and only 1 by mammography. A total of 3 (1.0%) patients were detected through CBE: 1 didn’t undergo imaging screening and the other 2 were negative in the following ultrasound screening. But all 3 patients had positive imaging findings upon presentation at our hospital. A total of 5 (1.6%) patients were diagnosed incidentally by chest CT/MRI while evaluating another disease.
Table 1
| Mode of detection | Patient number (%) |
|---|---|
| Self-detection† | 225 (72.6) |
| Breast lump | 212 (95.1) |
| Nipple discharge | 22 (9.9) |
| Change in breast shape | 22 (9.9) |
| Nipple retraction | 20 (9.0) |
| Redness and/or swelling | 16 (7.2) |
| Axillary lump | 8 (3.6) |
| Ulceration | 6 (2.7) |
| Pruritus | 5 (2.2) |
| CBE | 3 (1.0) |
| Ultrasound screening | 76 (24.5) |
| Mammographic screening | 1 (0.3) |
| Chest CT/MRI | 5 (1.6) |
†, data on initial symptoms and signs of 2 self-detected patients were missing. CBE, clinical breast examination; CT, computed tomography; MRI, magnetic resonance imaging.
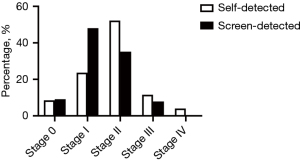
Mode of detection has been related to stage and prognosis of breast cancer (9). Consistent with previous studies, we found that screen-detected diseases were at earlier stages compared to self-detected diseases (Figure 1). But unexpectedly, there were still quite a few (32.2%) self-detected breast cancers diagnosed at stage 0–I, while the remaining 67.8% were at advanced stages (stage II–IV). Moreover, among the 3 patients that were detected by CBE, 2 had stage I diseases while the other had stage IV disease because she did not go visiting a doctor in 3 years until the tumor markedly increased in size.
Current situation of breast cancer screening
Age distribution and screening rates were depicted in Figure 2. Among total 310 patients, 166 (53.5%) had a history of breast cancer screening (ES). As illustrated in Figure 2, screening rate was higher in patients aged 59 and below and decreased with age, which was consistent with the fact that currently most screenings were provided by employers’ health check-up benefit and in some cases, they stopped providing it once the employee retired. For patients aged 40–69, who are the main target population of screening, screening rate was 57.4%.
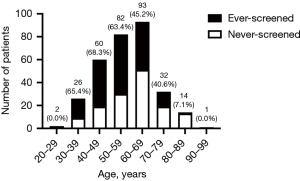
Table 2 shows the modality and frequency of previous screening in ES patients. The most common screening approach used was ultrasonography: 129 (77.7%) with ultrasonography alone and 32 (19.3%) with ultrasonography combining mammography. A total of 5 (3.0%) patients chose mammography as the sole screening tool. Since some screenings were provided by employers and others were actions of individuals, not all screenings were regular (annual or biennial). In total, 40 (24.1%) patients underwent occasional screening, 98 (59.0%) patients took organized ultrasound screening alone, 19 (11.5%) patients also took occasional mammographic screening, and only 9 (5.4%) were regularly screened by ultrasonography as well as mammography.
Table 2
| Ultrasonography | Mammography | |||
|---|---|---|---|---|
| Never | Occasional† | Biennial | Annual | |
| Never | 144 | 5 | 0 | 0 |
| Occasional† | 31 | 4 | 0 | 0 |
| Biennial | 11 | 3 | 1 | 0 |
| Annual | 87 | 16 | 3 | 5 |
†, screening interval longer than 2 years or had screened once only.
Self-identified and demographic barriers to screening
When asked the reason why they did not participate in breast cancer screening, 99 (68.8%) NS patients said that they had little knowledge of breast cancer screening before. A total of 43 (30.0%) complained about lack of access to screening, since they were unemployed or breast cancer screening was not covered by their employers’ health check-up benefit. And other barriers to screening included low perceived susceptibility (1.4%) and the perception that screening is unnecessary (1.4%).
To further understand factors contributing to disparities in screening, we compared demographic features of ES and NS patients. ES patients had a younger age and a lower BMI compared to NS patients (Table 3). Accordingly, there was a higher percentage of pre-menopause patients in ES group than NS group. ES patients were more likely to receive higher education, reside in urban areas, be currently working, and have higher family income, indicating that disparity of screening is closely associated with socioeconomic status. No difference was observed in marital status, family history of breast cancer and history of benign breast diseases or other malignancy between the two groups.
Table 3
| Characteristics | NS | ES | P value |
|---|---|---|---|
| Number | 144 (46.5) | 166 (53.5) | |
| Age, mean (SD), years | 60.9 (13.6) | 54.5 (10.9) | 0.000 |
| BMI, mean (SD), kg/m2 | 25.3 (3.6) | 24.0 (3.3) | 0.002 |
| Marital status | 0.189 | ||
| Unmarried | 3 (2.1) | 4 (2.4) | |
| Married | 125 (86.8) | 152 (91.6) | |
| Divorced/separated/widowed | 16 (11.1) | 9 (5.4) | |
| Unknown† | 0 (0.0) | 1 (0.6) | |
| Menopause | 0.006 | ||
| No | 33 (22.9) | 62 (37.3) | |
| Yes | 111 (77.1) | 104 (62.7) | |
| Family history | 0.110 | ||
| No | 133 (92.4) | 144 (86.7) | |
| Yes | 11 (7.6) | 22 (13.3) | |
| Benign breast disease history | 0.922 | ||
| No | 134 (93.1) | 154 (92.8) | |
| Yes | 10 (6.9) | 12 (7.2) | |
| History of other malignancy | 0.610 | ||
| No | 122 (84.7) | 144 (86.7) | |
| Yes | 22 (15.3) | 22 (13.3) | |
| Type of insurance | 0.009 | ||
| Rural | 26 (18.1) | 12 (7.2) | |
| Urban | 106 (73.6) | 145 (87.3) | |
| Others | 10 (6.9) | 9 (5.4) | |
| Unknown† | 2 (1.4) | 0 (0.0) | |
| Education level | 0.000 | ||
| Primary and below | 38 (26.4) | 11 (6.6) | |
| Secondary | 62 (43.1) | 66 (39.8) | |
| Higher | 44 (30.6) | 89 (53.6) | |
| Working status | 0.024 | ||
| No work | 22 (15.3) | 16 (9.6) | |
| Currently working | 32 (22.2) | 59 (35.5) | |
| Retired | 90 (62.5) | 91 (54.8) | |
| Yearly family income, yuan | 0.002 | ||
| ≤70,000‡ | 66 (45.8) | 56 (33.7) | |
| 70,000–140,000 | 45 (31.2) | 49 (29.5) | |
| 140,000–210,000 | 24 (16.7) | 24 (14.5) | |
| >210,000 | 7 (4.9) | 31 (18.7) | |
| Unknown† | 2 (1.4) | 6 (3.6) |
Data are presented as number (percentage) unless otherwise stated. †, not included in the statistical analysis; ‡, per-capita disposable income in Beijing was 69,434 yuan in 2020. NS, never-screened; ES, ever-screened; SD, standard deviation; BMI, body mass index.
Discussion
While most guidelines for breast cancer screening recommend annual or biennial mammography for women aged 40–69 (10,11), screening in China is predominantly based on ultrasonography. There could be many reasons: insufficient cost-effectiveness data in Chinese population, worries about potential false negative results in women with dense breasts, patients’ negative experience during the procedure, and most importantly, shortage of mammography equipment and high cost which is about twice the price of ultrasonography and not covered by statutory health insurance (12,13).
However, effectiveness of ultrasound screening has long been disputed. Although a meta-analysis of 11 studies comparing the efficacy of mammography and ultrasonography in screening found no significant differences in sensitivity, specificity and cancer-detection rate (13), there are still concerns because accuracy of ultrasonography highly relies on training and experiences of the operator. Results from the 2008 Chinese National Breast Cancer Screening Program have demonstrated a considerable impact of ultrasound-based screening on early detection of breast cancer in rural women (6). But only CBE positive individuals were included for screening and there was no proper unscreened control in that study.
Nevertheless, one study summarized randomized breast screening trials and found that whether there was mortality reduction largely depends on the success of the screening program (14). In trials that achieved 20% or greater reduction in advanced-stage disease, reduction in breast cancer mortality were significant (28% on average), but in those with a 10% or less reduction in advanced-stage disease, no reduction in cancer mortality was observed. In light of this, our result that there was a more than 20% reduction in stage II or more advanced breast cancer in screen-detected patients compared to the self-detected group might predict a long-term survival benefit from an ultrasound-based screening. In the short term, on the other hand, screening may reduce treatment-related impairment. For instance, a smaller tumor size increases the chance of breast conserving surgery, while lymph node dissection and associated lymphoedema might be averted if lymph node has not been involved at treatment.
World Health Organization recommended a minimal screening coverage of 70% to reduce breast cancer mortality (15). However, in the absence of nationwide screening programs in China, most screenings are opportunistic. In our study, only 57.4% of screening-eligible individuals had ever undergone screening and the true screening coverage is likely to be lower in the general public. Screen-detected tumor accounted for less than 25% in total. Although this rate has increased compared to 10 years ago (5.2%) (16), there is still a huge gap to be bridged when compared with the USA (7,8).
Despite potentially considerable benefit, population-based imaging screening in China is challenging which requires large numbers of personnel and equipment. Current screenings are mostly provided by employers’ health check-up benefit and thus small in scale. Accordingly, unemployment, retirement, jobs that do not offer screenings are all barriers cutting off women’s most likely access to screening as public awareness is insufficient to drive spontaneous screening on an individual level, which requires not only knowledge but also money. As such, social deprivation could at least partially explain the disparity in screening behavior today. Altogether, our results support a widespread implementation of organized, invitational and free ultrasound screening for the general public, especially the social deprived group, while mammography could be provided as an alternative or supplement in well-equipped facilities for those who can afford.
Given the practical dilemma of enhancing screening coverage in a short term, BSE and CBE might be considered more feasible screening tools though a lot more controversies. Results from meta-analysis of observational studies and clinical trials showed that current evidences do not support using BSE as a screening method (17). A large randomized trial in Shanghai also showed that BSE failed to reduce the incidence of late-stage breast cancer as well as cancer mortality but increased detection of benign breast diseases and biopsy rates (18). A cross-sectional study consisting of 886 breast cancer patients in Mexico indicated that identification through symptoms instead of screening was the most important risk factor for stage III–IV diseases (19). However, a recent study in the UK reported that breast lump alone and together with other symptoms were respectively reported in 97% and 88% of breast cancer patients with stage I–III diseases (20). In keeping with this, our results that self-detected breast cancers were mostly at stage II or earlier and about one third were actually identified at early stages suggested that self-detection is not too late as thought. Since most self-detected patients had a palpable breast lump, tutorials for proper BSE and campaigns advertising ‘a painless breast lump’ as an alert symptom for breast cancer might be of help.
Evidence of the clinical value of CBE varies in studies from developed countries and underdeveloped countries (5,21,22). In Canada, one study compared the effects of mammography combining CBE with CBE alone in reducing breast cancer mortality and no difference was observed (21). However, another study from Peru, where screening coverage is relatively low, demonstrated that CBE served as a screening and educational tool which shortened patient delay and reduced risk of advanced disease (5). A large, prospective, randomized controlled trial conducted in Mumbai also showed that patients with CBE were detected at earlier stages and had reduced mortality compared to controls (22). Consistently, our results showed that 2 of the 3 CBE-detected patients who presented in time had stage I diseases. This again proves that physical examination might not be necessary in countries with widely practiced mammographic screening but could still play an essential role in resource-starved areas.
Several limitations of this study should be noted. First, screenings of our patients were opportunistic and there was no electronic record which we could trace, so screening behavior was evaluated based on patients’ memory. This may introduce recall bias, especially for those who were diagnosed several years ago. However, the results such as mode of detection from medical records and our interviewers were consistent, adding to the reliability of patients’ answers. Second, the fact that samples were all from one single, newly established (opened since the end of 2014) medical facility in Beijing limits the generalizability of this study. For example, mean age of our cohort was 57.5 years, which is older than the general breast cancer population in China (23).
Conclusions
In conclusion, we showed that self-detection remains the most common way of breast cancer detection even in the most developed city in China. Yet, late-stage breast cancers were uncommon. Participation in breast cancer screening is still not satisfactory in the absence of nation-wide screening programs, and to solve that, raising public awareness and ensuring accessible screening and diagnostic resources are of equal importance and urgency.
Acknowledgments
Funding: This work was supported by the Beijing Municipal Health System High-Level Health Technical Talent Training Program (No. 2013-2-032 to BL).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-2/rc
Data Sharing Statement: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-2/dss
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Ethics Committee of Tsinghua Changgung Hospital (No. 21381-0-01). Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen C, Sun S, Yuan JP, et al. Characteristics of breast cancer in Central China, literature review and comparison with USA. Breast 2016;30:208-13. [Crossref] [PubMed]
- Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. [Crossref] [PubMed]
- Nelson HD, Tyne K, Naik A, et al. Screening for Breast Cancer: Systematic Evidence Review Update for the US Preventive Services Task Force. Rockville: Agency for Healthcare Research and Quality (US); 2009.
- Tabár L, Dean PB, Chen TH, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2019;125:515-23. [Crossref] [PubMed]
- Romanoff A, Constant TH, Johnson KM, et al. Association of Previous Clinical Breast Examination With Reduced Delays and Earlier-Stage Breast Cancer Diagnosis Among Women in Peru. JAMA Oncol 2017;3:1563-7. [Crossref] [PubMed]
- Huang Y, Dai H, Song F, et al. Preliminary effectiveness of breast cancer screening among 1.22 million Chinese females and different cancer patterns between urban and rural women. Sci Rep 2016;6:39459. [Crossref] [PubMed]
- Malmgren JA, Parikh J, Atwood MK, et al. Impact of mammography detection on the course of breast cancer in women aged 40-49 years. Radiology 2012;262:797-806. [Crossref] [PubMed]
- Kaplan HG, Malmgren JA, Atwood MK, et al. Effect of treatment and mammography detection on breast cancer survival over time: 1990-2007. Cancer 2015;121:2553-61. [Crossref] [PubMed]
- Cedolini C, Bertozzi S, Londero AP, et al. Type of breast cancer diagnosis, screening, and survival. Clin Breast Cancer 2014;14:235-40. [Crossref] [PubMed]
- Schünemann HJ, Lerda D, Quinn C, et al. Breast Cancer Screening and Diagnosis: A Synopsis of the European Breast Guidelines. Ann Intern Med 2020;172:46-56. [Crossref] [PubMed]
- Siu ALU.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:279-96. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Yang L, Wang S, Zhang L, et al. Performance of ultrasonography screening for breast cancer: a systematic review and meta-analysis. BMC Cancer 2020;20:499. [Crossref] [PubMed]
- Tabár L, Yen AM, Wu WY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J 2015;21:13-20. [Crossref] [PubMed]
- World Health Organization. Cancer control: knowledge into action. WHO guide for effective programmes. Diagnosis and treatment. Geneva: World Health Organization, 2008.
- Yuan XM, Wang N, Ouyang T, et al. Current status of diagnosis and treatment of primary breast cancer in beijing, 2008. Chin J Cancer Res 2011;23:38-42. [Crossref] [PubMed]
- Hackshaw AK, Paul EA. Breast self-examination and death from breast cancer: a meta-analysis. Br J Cancer 2003;88:1047-53. [Crossref] [PubMed]
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002;94:1445-57. [Crossref] [PubMed]
- Unger-Saldaña K, Miranda A, Zarco-Espinosa G, et al. Health system delay and its effect on clinical stage of breast cancer: Multicenter study. Cancer 2015;121:2198-206. [Crossref] [PubMed]
- Koo MM, Swann R, McPhail S, et al. Presenting symptoms of cancer and stage at diagnosis: evidence from a cross-sectional, population-based study. Lancet Oncol 2020;21:73-9. [Crossref] [PubMed]
- Miller AB, To T, Baines CJ, et al. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000;92:1490-9. [Crossref] [PubMed]
- Mittra I, Mishra GA, Dikshit RP, et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. BMJ 2021;372: [Crossref] [PubMed]
- Wang Q, Li J, Zheng S, et al. Breast cancer stage at diagnosis and area-based socioeconomic status: a multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer 2012;12:122. [Crossref] [PubMed]
Cite this article as: Yang Y, Yu J, Bai Y, Liu A, Tian J, Guo L, Huo D, Zhao P, Ji W, Luo B. Self-detection remains a primary means of breast cancer detection in Beijing, China. Transl Breast Cancer Res 2023;4:27.