
Antibody-drug conjugate monotherapy refines the oncological efficacy as compared to therapy of physicians’ choices in advanced breast cancers: a systematic review and meta-analysis
Highlight box
Key findings
• Our results supported the administration of ADC monotherapy in the clinical setting of advanced breast cancers.
What is known and what is new?
• The growing appreciation of the rationality that antibody-drug conjugate (ADC) can be administrated as a single-agent regimen in patients, with a promising antitumor activity and manageable treatment-related adverse events (AEs).
• Advanced breast cancer patients administrated ADC monotherapy have an overall striking tumoricidal efficacy and tolerable toxicity when compared to TPCs. This novel therapy renders the similar tumor response, reduces disease progression, and prolongs OS; additionally, it achieves the similar frequency of any grade/grade ≥3 hematologic and non-hematologic toxicities.
What is the implication, and what should change now?
• It strictly recommends to elevate the clinical recommendations of ADC monotherapy in the treatment of advanced breast cancer patients.
Introduction
GLOBOCAN 2020 estimates uncover 19.3 million new cancer cases and nearly 10 million cancer-related deaths in 2020; with the aging and exploding of the population, the global cancer burden is portended to be 28.4 million cases in 2040 (1). In China, solid tumors are responsible for more than 70% of all cancer-related deaths (2). Strikingly, breast cancer overcomes the lung cancer as the most newly diagnosed cancers in female populations (1). A concomitant elevation in the diagnosis and decease of advanced breast cancers in the United States is observed during the past decade (1). Advanced breast tumors are becoming an intractable problem for oncologists worldwide and have caused a resounding social-economic-psychological burden.
The management of advanced breast tumors might be optimized by developing specific targeted agents because most available drugs can only achieve an unsatisfied survival. Antibody-drug conjugate (ADC) provides a novel therapeutic paradigm, which chemically tethers a payload (i.e., cytotoxic agent) with a monoclonal antibody directed to corresponding tumor-related antigen (3), and accordingly, the cytotoxic agent can be selectively delivered into tumor cells to maximize antitumor activity and minimize toxicity. Nine ADCs have currently been approved by the Food and Drug Administration for tumor treatment in clinical settings and more than 60 ADCs are been underway to evaluate their efficacy and safety (4).
Several ADCs (e.g., sacituzumab govitecan, glembatumumab vedotin, trastuzumab emtansine; lifastuzumab vedotin, rovalpituzumab tesirine, mirvetuximab soravtansine, trastuzumab deruxtecan) have demonstrated robust antitumor activity and acceptable safety profile in many phase 1/2 clinical studies of solid tumors (5-14). Many randomized controlled trials (RCTs) and meta-analysis demonstrated the better survival outcomes of ADC monotherapy as compared to therapy of physician’s choices (TPCs) in advanced solid tumor patients (15-17). Given these encouraging results, the landmark RCTs also evaluated the oncological efficacy and treatment-related adverse events (AEs) of ADC monotherapy in advanced breast tumors, with or without pretreatments (18-20). However, their conclusions are conflicting due to the administration of different ADCs and the diverse demographic characteristics of candidates. Therefore, we conducted the present meta-analysis to more precisely ascertain the role of ADC monotherapy in the treatment of patients with advanced breast tumors, regardless of the pretreating status. We present this article in accordance with the PRISMA reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-14/rc) (21,22).
Methods
This meta-analysis has been registered on PROSPERO (ID: CRD42023432893). There was no need for Ethical or Institutional Review Board Approval for the study design due to the nature of our work.
Literature search
A computerized literature retrieval was performed in the MEDLINE, Web of Science, Cochrane Library, Embase databases, and ClinicalTrials.gov to identify English published articles up to April 4th, 2023. The following terms were used: ((“cancer”[MesH] OR cancer OR tumor OR tumour OR carcinoma) AND (advanced OR metastatic)) AND (antibody-drug conjugate) AND (ORR OR (objective response) OR (overall response) OR PFS OR OS OR (progression-free survival) OR (overall survival)) AND (randomized controlled trials).
Inclusion and exclusion criteria
RCTs comparing the oncological efficacy and/or safety between advanced breast cancer patients received ADC monotherapy (categorized as the study cohort) and those received physician’s choice (categorized as the control cohort) were in full consideration for inclusion. Moreover, potential RCTs needed to meet the following inclusion criteria: (I) populations—advanced or metastatic breast carcinoma patients; (II) treatment strategy—single agent of approved ADC administrated in the study cohort and TPCs in the control cohort; and (III) endpoints—reported at least one of the following outcomes: objective response rate (ORR), clinical benefit rate (CBR), progression-free survival (PFS), overall survival (OS), and/or any grade AEs. Besides, it needed to remove the citations with any of the following conditions: (I) article type—reviews, case reports, prospective studies, retrospective studies, editorials, letters, comments, study protocols, and conference papers; (II) treatment strategy—other therapies combined in the study cohort, or ADC encompassed in the control cohort; and (III) overlapping study populations.
Data extraction and quality assessment
We extracted the following data from the included RCTs by using a standardized form: (I) study characteristics—clinical trial information, publication year, original nation, study phase, ADCs, and sample size of enrollment; (II) demographic characteristics—median age, primary tumor site, and race; and (III) outcome characteristics—the event number of ORR and CBR, the frequency of any grade/grade ≥3 AEs and the hazard ratio (HR) of PFS and OS. ORR was the accumulation of complete response and partial response according to Response Evaluation Criteria in Solid Tumors (RECIST), regardless of the version. CBR was defined as the percentage of patients who achieved complete response, partial response, or at least six months of stable disease. PFS was defined as the time from randomization to the time of first radiologic progression according to RECIST, or the date of death from any causes. OS was defined as the time from diagnosis to last follow-up or time of death. AEs were graded with the use of the National Cancer Institute Common Terminology Criteria for Adverse Events, regardless of the version. Quality assessment of all RCTs was judged by the Cochrane risk of bias tool with Review Manager 5.4 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). Two co-authors (Dr. Yuhua Song and Dr. Yang lv) independently executed the literature search, study selection, and data extraction. If there were any inconsistencies, they were resolved by discussion.
Data synthesis and statistical analysis
The primary outcome measure was odds ratio (OR) with the corresponding 95% confidential interval (CI) of ORR and CBR. The secondary outcome measure represented HR with 95% CI of PFS and OS and OR with 95% CI of the frequency of any grade/grade ≥3 AEs. The crude ORs were separately calculated and the crude HRs were extracted from the included articles. The number of events when was not provided in the papers was computed based on the endpoint percentage or other relevant information (e.g., the percentage of events and the total number). The heterogenicity that implicated the degree of variability in results across the analyzed studies was assessed by Cochran’s Q test and Higgins I2 statistic test (23); P<0.10 suggested significant heterogeneity, and different cutoff intervals of I2 values at 0–25%, 26–50%, 51–75%, and 76–100% mapped to nonsignificant, moderate, substantial, and considerable heterogeneity, respectively. A binary fixed-effect model, Mantel-Haenszel method or a binary random-effect model, Mantel-Haenszel method was used to pool the crude HRs or ORs in light of the heterogeneity test, namely the former for the meta-analysis with no significant heterogeneity (P≥0.10) and the latter for the meta-analysis with significant heterogeneity (24). We also conducted subgroup analysis of all the outcomes to explore possible causes of heterogeneity among study results. The publication bias was evaluated by an Egger’s test with a significant level of P<0.05. All statistical analysis was performed by the software StateSE, version 12.0 (https://www.stata.com/). The results of syntheses were visually displayed by the forest plots and those of the subgroup analyses were present by tables. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data were collected from randomized clinical trials, those trials have been approved by institutional review boards. Under this circumstance, the Affiliated Hospital of Qingdao University waived the requirement of ethical approval.
Results
Literature search
A PRISMA flow diagram of the literature screening selection was outlined in Figure 1. The study strategy yielded a total of 2,690 citations, and 309 reduplications, 473 conference papers, 131 reviews, 11 case reports, 12 study protocols, and eight letters were excluded. The remaining 1,746 potential citations were assessed by title and abstract screening, and 1,713 of them were removed; fundamental characteristics of the abstracts were judged with respect to the inclusion and exclusion criteria, and 33 full-length articles were obtained. After full-text scrutinization, 23 of them were further omitted by the following reasons: (I) non-ADC monotherapy in the study cohort (n=10); (II) overlapping study populations (n=5); (III) non-RCTs (n=3); (IV) single-arm trial (n=3); and (V) non-advanced solid tumor (n=2). Ultimately, 10 eligible RCTs (9,18-20,25-30) with 5,089 advanced breast tumor participants were involved in this meta-analysis.
Characteristics of the studies included for meta-analysis
Characteristics of the 10 included RCTs in the “study-level” analysis were shown in Table 1, and those in the “patient-level” analysis were summarized in Table 2. Phase 3 RCTs accounted for 80% of all studies; the publication year ranged from 2013 to 2023 (median: 2019); the median age in the study cohort and the control cohort both was 55 years old; the largest subset of original countries was the USA (n=9); trastuzumab emtansine ranked first for therapy in the study cohort (n=4); and five RCTs described patients with human epidermal growth receptor 2 (HER2)-positive breast cancer. Additionally, Table 1 provided the study names, the ClinicalTrials.gov identifiers, study phases, molecular subtypes, regimens for both cohorts, the numbers concerning events versus totals in the cohorts of ORR and CBR, and the crude HRs with 95% CIs of PFS and OS.
Table 1
| Study (NCT#) | Phase | Subtypes | Regimen of study group | Regimen of control group | Total samples | e/T of ORR (n/T) | e/T of CBR (n/T) | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study cohort | Control cohort | Study cohort | Control cohort | PFS | OS | ||||||||
| ASCENT (NCT02574455) | 3 | TNBC | Sacituzumab govitecan | TPCs | 468 | 82/235 | 11/233 | 163/235 | 73/233 | 0.41 (0.32–0.52) |
0.48 (0.38–0.59) |
||
| EMERGE (NCT01156753) | 2 | NA | Glembatumumab vedotin | TPCs | 124 | 10/83 | 5/41 | 51/83 | 24/41 | 1.19 (0.78–1.79) |
1.37 (0.85–2.17) |
||
| EMILIA (NCT00829166) | 3 | HER2+ | Trastuzumab emtansine | Capecitabine and lapatinib | 991 | 173/397 | 120/389 | NA | NA | 0.65 (0.55–0.77) |
0.68 (0.55–0.85) |
||
| METRIC (NCT01997333) | 2 | TNBC | Glembatumumab vedotin | Capecitabine | 327 | 29/218 | 15/109 | 112/218 | 42/109 | 0.95 (0.71–1.29) |
1.06 (0.78–1.43) |
||
| MARIANNE (NCT01120184) | 3 | HER2+ | Trastuzumab emtansine | Trastuzumab and taxane | 732 | 181/303 | 195/287 | 256/303 | 253/287 | 0.91 (0.73–1.13) |
0.86 (0.64–1.16) |
||
| TDM4450g (NCT00679341) | 2 | HER2+ | Trastuzumab emtansine | Trastuzumab and docetaxel | 137 | 42/67 | 41/70 | NA | NA | 0.59 (0.36–0.97) |
1.06 (0.48–2.35) |
||
| TH3RESA (NCT01419197) | 3 | HER2+ | Trastuzumab emtansine | TPCs | 602 | NA | NA | NA | NA | 0.53 (0.42–0.66) |
0.55 (0.37–0.83) |
||
| DESTINY-Breast02 (NCT03523585) | 3 | HER2+ | Trastuzumab deruxtecan | TPCs | 608 | NA | NA | NA | NA | 0.36 (0.28–0.45) |
0.66 (0.50–0.86) |
||
| TROPiCS-02 (NCT03901339) | 3 | Luminal HER2− | Sacituzumab govitecan | Chemotherapy | 543 | NA | NA | NA | NA | 0.66 (0.53–0.83) |
NA | ||
| DESTINY-Breast04 (NCT03734029) | 3 | HER2 low | Trastuzumab deruxtecan | TPCs | 557 | NA | NA | NA | NA | 0.50 (0.40–0.63) |
0.64 (0.49–0.84) |
||
NCT, normolized controlled trials; e/T, event/Toal; ORR, objective response rate; CBR, clinical benefit rate; CI, confidential interval; PFS, progression-free survival; OS, overall survival; TNBC, triple-negative breast cancer; NA, not available; HER2+/−, human epidermal growth receptor 2-positive/negative; TPC, treatment of physician’s choice.
Table 2
| Characteristics | Study (N=10), No. [%] | Analyzed participants (N=5,089), No. [%] |
|---|---|---|
| Study type | ||
| Phase 2 RCTs | 2 [20] | 588 [12] |
| Phase 3 RCTs | 8 [80] | 4,501 [88] |
| Publication year, median [range] | 2019 [2013–2023] | NA |
| Median age, median [range], years | ||
| Study cohort | 54 [52–65] | NA |
| Control cohort | 54 [52–66] | NA |
| Original nation | ||
| USA | 9 [90] | 4,098 [81] |
| Germany | 1 [10] | 991 [19] |
| Antibody-drug conjugate | ||
| Sacituzumab govitecan | 2 [20] | 1,011 [20] |
| Glembatumumab vedotin | 2 [20] | 451 [9] |
| Trastuzumab emtansine | 4 [40] | 2,462 [48] |
| Trastuzumab deruxtecan | 2 [20] | 1,165 [23] |
| Molecular subtypes | ||
| Triple-negative breast cancer | 2 [20] | 795 [16] |
| HER2+ breast cancer | 5 [50] | 3,070 [60] |
| HER2 low breast cancer | 1 [10] | 557 [11] |
| Luminal HER2− BC | 1 [10] | 543 [11] |
| Unclassified | 1 [10] | 124 [2] |
The calculation of median value is based on the provided data from included studies. RCT, randomized controlled trial; y, year; HER2+/−, human epidermal growth receptor 2-positive/negative; BC, breast cancer.
ORR and CBR
Overall, six RCTs with 2,808 unique participants provided available data for the assessment of tumor response (i.e., six for the analysis of ORR and four for that of CBR). The pooled effect on ORR was not statistically significant (OR =1.64; 95% CI: 0.86–3.13; P=0.136) (Figure 2A). With regard to the subgroup analysis of ADCs, ORR was significantly improved in sacituzumab govitecan, whereas the results in glembatumumab vedotin, and trastuzumab emtansine were nonsignificant (Table 3). Finally, no significantly improved ORR was observed in both phase 2 and 3 RCTs (Table 3).
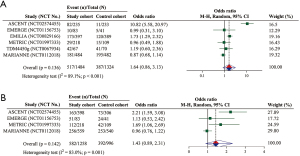
Table 3
| Subgroup analyses | No. of RCTs | Statistical results | Heterogeneity test | ||||
|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | Pooled P value | Weight (%) | I2 | P value | |||
| Objective response rate | |||||||
| ADCs | |||||||
| SG | 1 | 10.82 (5.58–20.97) | <0.001 | 16.50 | NA | NA | |
| GV | 2 | 0.97 (0.54–1.73) | 0.912 | 28.72 | 0.0 | 0.970 | |
| TE | 3 | 1.22 (0.74–2.02) | 0.444 | 54.78 | 82.7 | 0.003 | |
| Phase | |||||||
| III | 3 | 2.41 (0.85–6.84) | 0.097 | 54.99 | 96.1 | <0.001 | |
| II | 3 | 1.05 (0.68–1.64) | 0.816 | 45.01 | 0.0 | 0.904 | |
| Overall | 6 | 1.64 (0.86–3.13) | 0.136 | 100.00 | 90.6 | <0.001 | |
| Clinical benefit rate | |||||||
| ADCs | |||||||
| SG | 1 | 2.21 (1.59–3.08) | <0.001 | 27.89 | NA | NA | |
| GV | 2 | 1.51 (1.01–2.25) | 0.043 | 42.32 | 0.0 | 0.380 | |
| TE | 1 | 0.96 (0.76–1.21) | 0.726 | 29.80 | NA | NA | |
| Phase | |||||||
| III | 2 | 1.44 (0.64–3.28) | 0.379 | 57.68 | 93.9 | <0.001 | |
| II | 2 | 1.51 (1.01–2.25) | 0.043 | 42.32 | 0.0 | 0.380 | |
| Overall | 4 | 1.62 (0.79–3.34) | 0.142 | 100.00 | 83.0 | 0.001 | |
RCT, randomized controlled trial; OR, odds ratio; CI, confidence interval; ADC, antibody-drug conjugate; SG, sacituzumab govitecan; GV, glembatumumab vedotin; TE, trastuzumab emtansine; NA, not available.
The pooled result of CBR indicated no significant difference between the cohorts (OR =1.43; 95% CI: 0.89–2.31; P=0.142) (Figure 2B). As seen in Table 3, a significantly better outcome with CBR for the study cohort over the control cohort was observed in the subgroup analysis of sacituzumab govitecan and glembatumumab vedotin, but not in that of trastuzumab emtansine (Table 3). Moreover, phase 2 RCTs showed a significantly higher CBR in the study cohort than the control cohort, although no significant difference was observed in phase 3 RCTs (Table 3).
PFS and OS
Overall, all RCTs were included for the evaluation of survival (i.e., 10 for the analysis of PFS and 9 for that of OS). The pooled results showed a significant superiority in PFS of the study cohort relative to the control cohort (HR =0.62; 95% CI: 0.50–0.74; P<0.001) (Figure 3A). The subgroup analyses of ADCs in sacituzumab govitecan, trastuzumab emtansine, and trastuzumab deruxtecan revealed a significantly better PFS in the study cohort than the control cohort (Table 4). Moreover, PFS was significantly improved in phase 2 RCTs but marginally improved in phase 3 RCTs (Table 4).
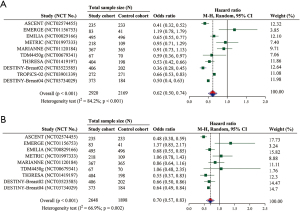
Table 4
| Subgroup analyses | No. of RCTs | Statistical results | Heterogeneity test | ||||
|---|---|---|---|---|---|---|---|
| Pooled HR (95% CI) | Pooled P value | Weight (%) | I2 | P value | |||
| Progression-free survival | |||||||
| ADCs | |||||||
| SG | 2 | 0.53 (0.28–0.77) | <0.001 | 23.40 | 86.5 | 0.007 | |
| GV | 2 | 1.01 (0.76–1.26) | 0.930 | 11.26 | 0.0 | 0.419 | |
| TE | 4 | 0.66 (0.51–0.81) | <0.001 | 40.98 | 71.0 | 0.016 | |
| TD | 2 | 0.47 (0.27–0.67) | <0.001 | 10.10 | 0 | 0.876 | |
| Phase | |||||||
| III | 7 | 0.56 (0.44–0.68) | <0.001 | 81.68 | 85.5 | <0.001 | |
| II | 3 | 0.87 (0.55–1.19) | 0.384 | 18.32 | 59.8 | 0.083 | |
| Overall | 10 | 0.62 (0.50–0.73) | <0.001 | 100.00 | 84.2 | <0.001 | |
| Overall survival | |||||||
| ADCs | |||||||
| SG | 2 | 0.55 (0.38–0.73) | <0.001 | 32.20 | 65.1 | 0.090 | |
| GV | 2 | 1.12 (0.83–1.41) | 0.335 | 12.12 | 0.0 | 0.409 | |
| TE | 4 | 0.69 (0.56–0.83) | <0.001 | 40.98 | 18.5 | 0.298 | |
| TD | 1 | 0.64 (0.47–0.81) | <0.001 | 14.70 | NA | NA | |
| Phase | |||||||
| III | 6 | 0.62 (0.52–0.73) | <0.001 | 86.13 | 53.4 | 0.057 | |
| II | 3 | 1.12 (0.84–1.39) | 0.304 | 13.87 | 0.0 | 0.706 | |
| Overall | 9 | 0.70 (0.57–0.83) | <0.001 | 100.00 | 66.9 | 0.002 | |
RCT, randomized controlled trial; HR, hazard ratio; CI, confidence interval; ADC, antibody-drug conjugate; SG, sacituzumab govitecan; GV, glembatumumab vedotin; TE, trastuzumab emtansine; TD, trastuzumab deruxtecan; NA, not available.
The pooled result of OS indicated a significant improvement in the study cohort (HR =0.70; 95% CI: 0.57–0.83; P<0.001) (Figure 3B). Consistent to the subgroup analysis of PFS, the significant better results were observed in sacituzumab govitecan, trastuzumab emtansine, and trastuzumab deruxtecan (Table 4). The subgroup analysis of phase 2 RCTs was also statistically significant (Table 4).
Frequency of any grade/grade ≥3 AEs
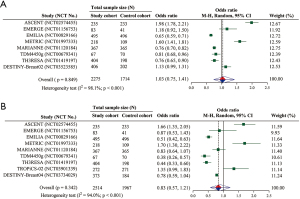
Overall, nine included RCTs provided data for analyzing frequency of AEs, with 4,481 unique participants. The pooled OR suggested that the frequency of any grade AEs (OR =1.03; 95% CI: 0.75–1.41; P=0.849) and that of grade ≥3 AEs (OR =0.83; 95% CI: 0.57–1.21; P=0.342) was both not significantly different between the cohorts (Figure 4A,4B).
We further performed a subgroup analysis of ADCs according to hematologic and non-hematologic toxicity, respectively (Table S1). A significantly higher frequency of any grade hematologic toxicity was observed in sacituzumab govitecan but in glembatumumab vedotin and trastuzumab emtansine. The frequency of any grade non-hematologic toxicity was significantly increased in sacituzumab govitecan and glembatumumab vedotin, but significantly decreased in trastuzumab emtansine. Furthermore, sacituzumab govitecan was more frequent to develop grade ≥3 hematologic toxicities than TPCs. Finally, a significantly greater frequency of grade ≥3 non-hematologic toxicity was observed in sacituzumab govitecan and glembatumumab vedotin.
Publication bias and sensitivity analysis
The publication bias of all meta-analyses in terms of the P value (ranging from 0.137 to 0.704) was statistically nonsignificant (Figure S1, Table S2), indicating no existence of publication bias. Sensitivity analysis was utilized for selecting appropriate studies in the individual meta-analysis (Figures S2-S7) (31).
Quality appraisal and evidence level
The high risk of bias was over 50% in the domains including allocation concealment, blinding of participants and personnel, and blinding of outcome assessment, whereas no high risk of bias was observed in the following domains, random sequence generation, incomplete outcome data, selective reporting, and other bias (Table S3). Finally, all analyses only showed a moderate level of evidence (Grade evidence by GRADEpro system, Figure S8).
Discussion
The present study included 10 phase 2/3 RCTs of ADC monotherapy for patients with advanced breast tumors, wherein the control arms comprised of mono-chemotherapy, polychemotherapy, and dual-targeted therapy; our results reflected the oncological efficacy and safety profile of ADC monotherapy versus the most common physician’s choice in relevant advanced solid tumors. The meta-analysis demonstrates the significant correlation between ADC monotherapy and the refined oncological efficacy, with an overall decrement of 22% in the instantaneous risk of disease progression and that of 36% in the risk of survival threat in this clinical setting. In addition, ADC monotherapy appears an overall alike frequency of any grade treatment-related AEs and grade ≥3 AEs to the standard-of-care in advanced cancer tumors.
As a steeply evolving therapeutic modality, ADC harbors the monoclonal antibody that functions as a vehicle directly carrying the payload to tumors, which theoretically experiences a stronger anticancer activity and achieves more tumor shrinkage. Results from our study did not show the significant difference of ORR and CBR between ADC monotherapy and TPCs in patients with advanced breast tumors. By contrast, when changing regimens of the control cohort from capecitabine plus lapatinib towards trastuzumab plus taxane, the inferiority of ADC monotherapy in HER2-positive breast cancer arises, yet the CBR is still not significantly different (19). These interesting findings contribute to the phase 3 MARIANNE study (ClinicalTrials.gov identifier NCT01120184) (19) further investigating tumor response between ADC (i.e., trastuzumab emtansine) combined with targeted therapy (i.e., pertuzumab) and trastuzumab combined with taxane in advanced HER2-positive breast cancers. Although ORR in the trastuzumab emtansine plus pertuzumab arm does not outnumber the trastuzumab plus taxane arm (64.2% vs. 67.9%), the median duration of response (DoR) in the former is significantly higher than the latter (21.2 vs. 12.5 months); furthermore, single-agent of trastuzumab emtansine fulfills a discernable increment of median DoR as compared to trastuzumab plus taxane. A drawback of our study was that the analysis of DoR between the cohorts was not conducted due to the insufficient data from included RCTs.
The ultimate goal of clinical treatment is to lengthen the duration of survival. The survival benefits are not observed in all ADCs; specifically, the administrations of trastuzumab emtansine in HER2-positive breast cancers and sacituzumab govitecan in triple-negative breast cancer (TNBC) all benefit the improvements in PFS and OS (Figure 3A,3B). Despite that the overall ameliorated PFS and OS of ADC monotherapy in advanced breast tumors is demonstrated by our study, we should perceive the dramatically different situations caused by the diversity of tumor ecosystem and ADCs.
Our results implicate the overall similar frequency of any grade/grade ≥3 AEs between ADC monotherapy and physician’s choice. Furthermore, with reference to the overall frequency of any grade/grade ≥3 hematologic and non-hematologic toxicity, there is still no significant difference between the cohorts. In fact, as the crude OR values are shown in Figure 4A,4B, the frequency comparison of any grade AEs or grade ≥3 AEs between the cohorts in per-RCTs is discordant due to the diversity of ADC in the study cohort and the regimens in the control cohort.
The majority of four included RCTs (9,20,26), excepting the phase 3 MARIANNE study (19), collectively denote the significantly lower frequency of any grade/grade ≥3 AEs in advanced HER2-positive breast cancer patients treated with trastuzumab emtansine monotherapy than those treated with physician’s choice. In TNBC, the occurrence of low-grade/grade ≥3 AEs of two ADCs (sacituzumab govitecan, and glembatumumab vedotin) is more frequent than physician’s choice; the most clinically noteworthy AEs in sacituzumab govitecan are neutropenia and diarrhea and in glembatumumab vedotin are rash, peripheral neuropathy and alopecia (25,27). Importantly, the frequency of any grade AEs in advanced ovarian cancer patients undergoing mirvetuximab soravtansine is significantly higher than those undergoing physician’s choice, whilst that of grade ≥3 AEs is lower in the mirvetuximab soravtansine arm.
There are some limitations in this first meta-analysis regarding ADC monotherapy in advanced solid tumors. The first limitation of our study is that all pooled results are calculated by a random effect model and are manifested with considerable heterogeneity; however, the leave-one-out sensitivity analyses guarantee the reasonable inclusion of studies for individual meta-analyses. The heterogeneity was likely correlated to the design of the study itself because RCTs with different phases, therapy lines, ADCs, agents in TPCs, and molecular subtypes were involved for analysis. Second, subgroup analysis is neither conducted in terms of treatment lines nor with or without the previous chemotherapy, which is attributive to the inadequate information provided from partially analyzed RCTs.
Conclusions
ADC monotherapy demonstrates the overall improvements in survival benefits plus the overall comparable frequency of AEs in advanced breast tumors when compared to TPCs. However, we could not use an ‘one-size-fits-all’ management approach to supersede physician’s choice by ADC monotherapy in all patients of advanced breast tumor, because these clinical benefits and similar safety profiles between the two arms experienced dramatically different results across all analyzed RCTs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-14/rc
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-14/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-14/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data were collected from randomized clinical trials, those trials have been approved by institutional review boards. Under this circumstance, the Affiliated Hospital of Qingdao University waived the requirement of ethical approval.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl 2014;53:3796-827. [Crossref] [PubMed]
- Birrer MJ, Moore KN, Betella I, et al. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J Natl Cancer Inst 2019;111:538-49. [Crossref] [PubMed]
- Starodub AN, Ocean AJ, Shah MA, et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin Cancer Res 2015;21:3870-8. [Crossref] [PubMed]
- Bendell J, Saleh M, Rose AA, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol 2014;32:3619-25. [Crossref] [PubMed]
- Ott PA, Hamid O, Pavlick AC, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with advanced melanoma. J Clin Oncol 2014;32:3659-66. [Crossref] [PubMed]
- Krop IE, Suter TM, Dang CT, et al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol 2015;33:1136-42. [Crossref] [PubMed]
- Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2013;31:1157-63. [Crossref] [PubMed]
- Burris HA, Gordon MS, Gerber DE, et al. A phase I study of DNIB0600A, an antibody-drug conjugate (ADC) targeting NaPi2b, in patients (pts) with non-small cell lung cancer (NSCLC) or platinum-resistant ovarian cancer (OC). Journal of Physical Chemistry A 2014;102:3127-33.
- Morgensztern D, Besse B, Greillier L, et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin Cancer Res 2019;25:6958-66. [Crossref] [PubMed]
- Moore KN, Martin LP, O'Malley DM, et al. A review of mirvetuximab soravtansine in the treatment of platinum-resistant ovarian cancer. Future Oncol 2018;14:123-36. [Crossref] [PubMed]
- Shitara K, Iwata H, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol 2019;20:827-36. [Crossref] [PubMed]
- Burris HA 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011;29:398-405. [Crossref] [PubMed]
- Zhang L, Shen D, Yu L, et al. Is antibody-drug conjugate a rising star for clinical treatment of solid tumors? A systematic review and meta-analysis. Crit Rev Oncol Hematol 2022;177:103758. [Crossref] [PubMed]
- Moore KN, Oza AM, Colombo N, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol 2021;32:757-65. [Crossref] [PubMed]
- Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020;382:2419-30. [Crossref] [PubMed]
- Yardley DA, Weaver R, Melisko ME, et al. EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer. J Clin Oncol 2015;33:1609-19. [Crossref] [PubMed]
- Perez EA, Barrios C, Eiermann W, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J Clin Oncol 2017;35:141-8. [Crossref] [PubMed]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [Crossref] [PubMed]
- McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. [Crossref] [PubMed]
- Liang Z, Zhang Q, Wang C, et al. Hyaluronic acid/ Hyaluronidase as biomarkers for bladder cancer: a diagnostic meta-analysis. Neoplasma 2017;64:901-8. [Crossref] [PubMed]
- Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. XXXX: John Wiley; 2000.
- Vahdat LT, Schmid P, Forero-Torres A, et al. Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer ("METRIC"): a randomized multicenter study. NPJ Breast Cancer 2021;7:57. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 2021;384:1529-41. [Crossref] [PubMed]
- André F, Hee Park Y, Kim SB, et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 2023;401:1773-85. [Crossref] [PubMed]
- Rugo HS, Bardia A, Marmé F, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol 2022;40:3365-76. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010;1:112-25. [Crossref] [PubMed]
Cite this article as: Song Y, Lv Y. Antibody-drug conjugate monotherapy refines the oncological efficacy as compared to therapy of physicians’ choices in advanced breast cancers: a systematic review and meta-analysis. Transl Breast Cancer Res 2023;4:11.


