
Prospect of neoadjuvant/adjuvant immunotherapy in early-stage triple-negative breast cancer
Introduction
In 2020, breast cancer overtook lung cancer as the most diagnosed cancer worldwide, with over 2 million cases diagnosed each year. China is also transitioning from a growing breast cancer burden, with new cases increasing from 0.3 million in 2015 to 0.42 million in 2020, accounting for 18% of all breast cancer cases globally (1). Triple-negative breast cancer (TNBC) is defined as breast cancer that lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounting for approximately 15% to 20% of all breast cancers. TNBC is more aggressive, prone to early recurrence and metastasis, and has a poor prognosis with a 5-year survival rate of less than 15%. However, after 5 years, the recurrence rate decreased significantly and was even lower than that of other breast cancer subtypes after 8 years (2). As a result, improving the prognosis of TNBC remains a challenge in the treatment of breast cancer. Additionally, effective treatment of TNBC at an early stage would significantly improve the chances of tumor resection and breast preservation for patients. Regardless of molecular heterogeneity, the standard systemic treatment for TNBC is similar to that for other types of breast cancer, which means neoadjuvant (preoperative) and/or adjuvant (postoperative) chemotherapy remains a critical component of systemic treatment for TNBC. Among them, neoadjuvant chemotherapy is used to shrink the size and stage of the tumor, allowing TNBC patients to achieve improved pathologic complete response (pCR) and long-term benefits, such as event-free survival (EFS) and overall survival (OS). Simultaneously, the sensitivity of the tumor to the chemotherapeutics can be determined to guide subsequent adjuvant treatment. While adjuvant chemotherapy is beneficial for eradicating residual lesions, preventing tumor recurrence and metastasis, and consolidating the results of neoadjuvant chemotherapy and surgery.
Immunotherapy is bringing benefit for early-stage operable cancer patients in neoadjuvant/adjuvant treatment. The advent of immunotherapy in the form of immune checkpoint inhibitors (ICIs) expands the treatment options in multiple early stage cancers, such as the application of neoadjuvant immunotherapy in non-small cell lung cancer (NSCLC) and adjuvant immunotherapy in esophageal or gastroesophageal junction (GEJ) cancer, urothelial carcinoma (UC) and NSCLC. Early stage TNBC treatment is at a bottleneck due to the limited efficacy of traditional neoadjuvant/adjuvant chemotherapy, such as only about 30% pCR in neoadjuvant chemotherapy setting (2). The application of neoadjuvant/adjuvant immunotherapy for early stage TNBC has been proved to improve the pCR as well as EFS in clinical trials (3-6). On July 27, 2021, FDA approved pembrolizumab for treatment of patients with high-risk early-stage TNBC in combination with chemotherapy as neoadjuvant treatment and then continued as a single agent as adjuvant treatment following surgery. PD-1 inhibitors are also recommended for early-stage TNBC in the Chinese Society of Clinical Oncology’s (CSCO) breast cancer guidelines published in 2022, in which the level III recommendation includes “chemotherapy plus PD-1 inhibitor” (7). In this review, we will combine CSCO guidelines and clinical practice in China to discuss the progress of neoadjuvant/adjuvant immunotherapy in managing early-stage TNBC, as well as potential challenges and strategies for improving clinical outcomes. Additionally, we present our cTRIO clinical trial for Chinese patients with early-stage TNBC that synthesized prior research experiences.
The rationality of immunotherapy in combination with chemotherapy for early-stage TNBC
The ability of various types of tumors to induce immune responses varies significantly. “Inflamed tumors” or “hot tumors” have been used to describe malignant tumors with a high level of immunogenicity (8). Clinical evidence is mounting that ICIs targeting programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) are the most effective treatment for these “inflamed tumors”. TNBC is more immunogenic than the other breast cancer subtypes and is generally considered to be the breast cancer subtype that most closely resembles “inflamed tumors”. For example, TNBC is found with high mutation rate and is capable of producing tumor-specific neoantigens that mediate immune responses. TNBC also expresses a higher level of PD-L1, providing a target for ICI immunotherapy (9,10). Additionally, previous studies showed that early-stage TNBC is more immunogenic compared to advance TNBC due to higher immune cell infiltration. Taken together, these characteristics indicate that early-stage TNBC is an excellent candidate for immunotherapy, particularly in patients who have never received chemotherapy, with relatively intact immune systems. However, monotherapy with ICIs achieved response in a small proportion of patients with TNBC, despite the potential for significant survival benefit once a response was achieved. For example, the objective response rate (ORR) of patients treated with atezolizumab in the first line was 26%. Thus, it is critical to increase the response rate of immunotherapies through combinational approaches (11).
Even though chemotherapy always compromises the immune system, preclinical and clinical studies have demonstrated synergy between cytotoxic drugs and immunotherapy (12). Chemotherapy has been shown to not only inhibit tumor growth, but also to increase the number of gene mutations associated neoantigen in tumor cells, transferring “cold tumor” to “hot tumor” by inducing a robust cytotoxic immune response in cancer models (13). Paclitaxel was found to induce macrophages to secrete pro-inflammatory cytokines, resulting in the recruitment and activation of dendritic cells and T cells. Chemotherapy can also inhibit myeloid-derived suppressor cells and FOXP3 regulatory T cells. Paclitaxel and cisplatin can increase the expression of mannose-6-phosphate receptors on tumor cells, thereby increasing granzyme B permeability. Anthracycline-treated tumor cells are particularly effective in eliciting an anticancer immune response, whereas other DNA-damaging agents such as etoposide and mitomycin C do not induce immunogenic cell death. That is one of the reasons KEYNOTE-522 chose additional doxorubicin or epirubicin based chemotherapy followed by TP with pembrolizumab. Additionally, several chemotherapeutics increase tumor cells’ susceptibility to natural killer (NK) cells by increasing the expression of the NKG2-D type II integral membrane protein. On the other hand, high TILs counts are associated with the high pCR to neoadjuvant chemotherapies in patients with primary breast cancers (14). As a result, the majority of clinical trials involving ICIs in early-stage TNBC have been conducted in combination with chemotherapy, owing to their capacity to promote an anti-tumor immune response. As we describe below, not only do these clinical studies validate our optimism for the expansion of immunotherapy from late stage to early stage TNBC, but they also contribute to the identification of the most successful strategies (Table 1).
Table 1
| Item | KEYNOTE-522 (Schmid et al., 2020) | IMpassion031 (Mittendorf et al., 2020) | NeoTRIPaPDL1 (Gianni et al., 2019) | GeparNuevo (Loibl et al., 2019) |
I-SPY2 (Nanda et al., 2020) |
|---|---|---|---|---|---|
| Phase | 3 | 3 | 3 | 2 | 2 |
| Primary endpoint | pCR and EFS in ITT | pCR in ITT and in PD-L1+ patients | EFS | pCR in ITT | pCR in ITT |
| Patients enrolled | Untreated stage II–III TNBC patients | Untreated stage II–III TNBC patients | Untreated stage II–III TNBC patients | Untreated stage II–III TNBC patients | Untreated stage II–III HER2− BC patients |
| ICI | Pembro (anti-PD-1) | Atezo (anti-PD-L1) | Atezo (anti-PD-L1) | Durva (anti-PD-L1) | Pembro (anti-PD-1) |
| Platinum (carbo or cis) | Yes | No | Yes | No | No |
| Anthracycline | Yes | Yes (dd) | No | Yes (dd) | Yes (dd or non dd) |
| Taxane | Paclitaxel | Nab-Pac | Nab-Pac | Nab-Pac | Paclitaxel |
| Adjuvant | Pembro (or placebo) for 1 year. No capecitabine | Atezo (or placebo) for 1 year. Capecitabine allowed | Anthracyclines | Physician’s choice | Physician’s choice |
| No. of TNBC patients treated | 602 | 333 | 280 | 174 | 107 |
| Statistical outcome for pCR | Positive | Positive | Negative | Negative | Positive |
| pCR difference (Δ) in ITT | 14% | 17% | 3% | 9% | 38% |
| pCR Δ in PD-L1+ | 14% | 20% | 4% | 14% | NA |
| pCR Δ in PD-L1− | 15% | 14% | 0 | 33% | NA |
| Statistical outcome for EFS | Positive | NA | NA | NA | NA |
| HR for EFS | 0.63 (95% CI, 0.48–0.82) |
0.76 (95% CI, 0.40–1.44) |
NA | NA | 0.60 (95% CI, NA) |
| Neoadjuvant: G ≥3 AEs | 76.8% | 63.4% | 77.5% | 64.1% | – |
| Adjuvant: G ≥3 AEs | 23.3% | – | – | – | – |
TNBC, triple-negative breast cancer; ICI, immune checkpoint inhibitor; pCR, pathologic complete response; ITT, intent-to-treat; PD-L1, programmed death-ligand 1; EFS, event-free survival; HR, hazard ratio; G, grade; AEs, adverse events; PD-1, programmed death-1; CI, confidence interval; dd, dose-dense; Nab-Pac, nanoparticle albumin–bound paclitaxel; y, year; NA, not available; HER2, human epidermal growth factor receptor 2; BC, breast cancer.
Strategies to improve the clinical benefits
ICIs in neoadjuvant and/or adjuvant phase
ICIs, like chemotherapy, can be used during the neoadjuvant and/or adjuvant phases of the early-stage TNBC regimen. It was discovered that neoadjuvant pembrolizumab had a median OS nearly double that of adjuvant pembrolizumab (15). Additionally, a greater proportion of patients in the neoadjuvant arm had tumors with activated T-cell gene signatures than those in the adjuvant arm. Moreover, patients receiving neoadjuvant pembrolizumab had decreased expression of cell cycle–associated genes in tumors compared to patients receiving adjuvant pembrolizumab, implying that T cells activated by neoadjuvant ICIs may be more effective at inhibiting tumor cell aggressiveness. Thus, ICIs was used in the neoadjuvant phase of all early-stage TNBC immunotherapy trials (3-5). However, KEYNOTE-522 and IMpassion031 both used ICIs as adjuvant therapy, which may have been done to enhance the response to neoadjuvant therapy. Other studies exploring the efficacy of immunotherapy in adjuvant setting for those without neoadjuvant are also conducted.
Choice of ICI agents
Several mAbs were used in early-stage TNBC trials, including pembrolizumab for PD-1 blockade in I-SPY2 and KEYNOTE-522 (16,17), atezolizumab for PD-L1 blockade in NeoTRIP and IMpassion031 (18,19), and durvalumab for PD-L1 blockade in GeparNuevo (20). Theoretically, PD-1 and PD-L1 antibodies have comparable anti-tumor mechanisms, and it is widely believed that their clinical oncology efficacy and safety are equivalent. However, as related large-scale clinical research data have been released, the differences between the drugs have gradually been observed. Recently, Duan et al. used the “Mirror Principle” system to compare the efficacy and safety of PD-1 and PD-L1 mAbs in a meta-analysis (21). Among the studies on solid tumors included in the analysis, PD-1 mAbs administration resulted in a greater OS benefit than PD-L1 mAbs administration [hazard ratio (HR) =0.75; 95% confidence interval (95% CI), 0.65–0.86; P<0.001]. Moreover, this difference is more significant in combination immunotherapy (HR =0.68; 95% CI, 0.55–0.83; P<0.001) than in monotherapy (HR =0.78; 95% CI, 0.63–0.95; P=0.01). Additionally, because ICIs targeting PD-1 are more frequently used in clinical practice in China, we will use this type of mAb in our cTRIO trial.
Chemotherapy regimen
Taxanes and anthracyclines (TA) combinations have been widely used to treat TNBC. On the other hand, albumin-bound paclitaxel is more clinically concerned because it does not require hormone pretreatment and can increase the pCR rate of TNBC from 26% to 48% and the 3-year EFS rate from 80.7% to 87.1% when compared to traditional solvent-based paclitaxel (22). However, to avoid the cardiovascular AEs related to anthracyclines, the value of taxanes and platinum (TP) combinations has been gradually recognized, especially in China. Zhang et al. confirmed that the TP neoadjuvant chemotherapy regimen is superior to the standard TA combination for TNBC, with significantly improved pCR rate (38.6% vs. 14.0%, P=0.014) and 5-year RFS (77.6% vs. 56.2%, P=0.043) (23). Another phase III study for TNBC demonstrated that TP neoadjuvant chemotherapy with carboplatin plus albumin paclitaxel can significantly increase the pCR rate to 45.9%, with comparable 3-year EFS (24). As a result, both the NCCN and CSCO guidelines now recommend TP regimens for TNBC treatment.
pCR as surrogate endpoint
pCR refers to the absence of any viable tumor cells during resection. Numerous clinical studies have demonstrated that TNBC patients who achieve pCR through neoadjuvant chemotherapy have significantly longer EFS and OS than patients who do not achieve pCR. Because pCR was associated with an improved long term survival benefit following neoadjuvant chemotherapy in breast cancer, both NCCN and CSCO regulatory guidance supports the use of it as a surrogate primary endpoint in the neoadjuvant immunotherapy studies for early-stage TNBC. The results of KEYNOTE-522 indicated that the pCR rate for pembrolizumab plus chemotherapy was significantly higher than that for placebo plus chemotherapy in the ITT population (64.8% vs. 51.2%, pCR difference =14%, P=0.00055) (16). The benefits of pembrolizumab-chemotherapy with respect to the pCR were also observed consistently across other subgroups, including nodal status (positive or negative), tumor size (T1 to T2 or T3 to T4), and frequency of carboplatin administration (Q3W to QW). In IMpassion031, compared with the placebo group, the absolute benefit of atezolizumab combined with chemotherapy for pCR was as high as 16.5% (57.6% vs. 41.1%, P=0.0044) (18). Additionally, the benefit of pCR was observed consistently across other subgroups, including those with ECOG score 0 or 1 and Phase II vs. Phase III defined by baseline characteristics. Unfortunately, the NeoTRIP results showed that the pCR rate was 43.5% (95% CI, 35.1–52.2%) with atezolizumab and 40.8% (95% CI, 32.7–49.4%) without atezolizumab in the ITT population, resulting in an odds ratio of 1.11 (95% CI, 0.69–1.79; P=0.066), indicating that atezolizumab did not significantly improve the efficacy of neoadjuvant chemotherapy (19). The negative outcome may be explained by relatively high-risk baseline disease characteristics and the late stage of disease (positive lymph nodes accounted for 87%) of the study subjects. Perhaps another reason is that the study’s endpoint is EFS rather than pCR.
Long-term survival benefit
The primary benefit of immunotherapy is to improve long-term survival, even in some non-pCR populations. The most recent data from the KEYNOTE-522 study demonstrated that pembrolizumab plus chemotherapy significantly reduced the risk of EFS events by 37% (HR =0.63; 95% CI: 0.48–0.82; P=0.00031) over a median follow-up of 39 months (19). Additional follow-up of the OS revealed that pembrolizumab-chemotherapy reduced the risk of death by 28% (HR =0.72; 95% CI: 0.51–1.02; P=0.03214). The current official support of IMpassion031 for EFS, disease-free survival (DFS) or OS is not yet mature (19). Since in the all-randomized population, the time endpoint of long-term survival benefit did not reach the median to draw a clear conclusion. However, the observed HR for EFS (0.76; 95% CI, 0.40–1.44), DFS (0.74; 95% CI, 0.32–1.70), or OS (0.69; 95% CI, 0.25–1.87) all showed that atezolizumab plus chemotherapy group is beneficial. In GeparNuevo, after a median follow-up of 42.2 months, long-term survival data showed that invasive DFS (iDFS) was 92.0% and 71.9% in pCR and non-pCR populations, respectively (log-rank P=0.002), indicating that the benefits of pCR populations were more obvious (20). Additionally, durvalumab and placebo groups had a 3-year iDFS of 84.9% vs. 76.9% (HR =0.54; 95% CI, 0.27–1.09, stratified log-rank P=0.0559), a 3-year DDFS of 91.4% vs. 79.5% (HR =0.37; 95% CI, 0.15–0.87, P=0.0148), and a 3-year OS of 95.1% vs. 83.1% (HR =0.26; 95% CI, 0.09–0.79, P=0.0076), respectively. These findings demonstrated that the addition of durvalumab to neoadjuvant chemotherapy in early-stage TNBC significantly improved long-term outcome despite a small increase in pCR.
The impact of biomarkers
In TNBC, PD-L1 expression was estimated to be 40–65% on immune cells and 19% on tumor cells. The expression of PD-L1 has been investigated as a biomarker for predicting the response to ICI treatment and clinical studies indicated that ICIs in combination with chemotherapy is more likely to benefit patients with advanced TNBC who express PD-L1. In contrast, PD-L1 expression appears to have a less significant effect on the immunotherapy response in early-stage TNBC. In IMpassion130 for advanced and metastasis TNBC, the expression of PD-L1 was found predictive of prolonged PFS (HR =0.74; 95% CI, 0.61–0.91) and OS (HR =0.66; 95% CI, 0.50–0.88) with nab-paclitaxel in combination with atezolizumab vs. placebo (25). In KEYNOTE-355, the median PFS of PD-L1 positive patients with advanced metastasis TNBC was 7.6 months, which was significantly longer than the 5.6 months of chemotherapy alone (HR =0.74), and patients with a strong PD-L1 positivity (CPS10) had a more curative effect (26). However, as demonstrated in KEYNOTE-522 stratification analysis (3), both PD-L1 positive and negative patients can benefit from neoadjuvant immunotherapy, with a pCR difference of 14% in PD-L1+ patients and 15% in PD-L1 negative patients. EFS benefits were also not related to PD-L1 expression status, as determined by pre-designated exploratory subgroup EFS analysis. In the PD-L1 positive and negative subgroups, the risk of EFS events was reduced by 33% (HR =0.67) and 52% (HR =0.48), respectively. In IMpassion031, PD-L1 positive patients have the same pCR benefit as the ITT population (69% vs. 49%, rate difference 20%, 95% CI 4 to 35, one-sided P=0.021, did not cross the significance boundary P<0.0184). At the same time, even for PD-L1 negative patients, their pCR rate still has a trend of benefit (47.7% vs. 34.4%; rate difference 13%; 95% CI, −1 to 28). Of note, early studies showed the prognostic significance of tumor-infiltrating lymphocytes (TILs) in systemically untreated early TNBC, suggesting that the presence of TILs may refine the candidates for adjuvant chemotherapy or immunotherapy. Another study revealed that patients with early-stage TNBC who had circulating tumor cells (CTCs) positivity had a significantly worse DDFS, DFS and OS. Additional research is necessary to establish the potential utility of TIL and CTC as stratification biomarkers following neoadjuvant immunotherapy. Considering the safety, it is important to find a sensitive biomarker for those using checkpoint immunotherapy in neoadjuvant setting.
Safety profiles
The safety profile of the current trials is comparable across groups, and most of the adverse events (AEs) are related to chemotherapy and are consistent with previously reported AEs associated with mono-chemotherapeutics. However, it appears each immunotherapy has a unique safety profile. For KEYNOTE-522, the incidence of treatment-related adverse events (TRAEs) of grade 3 or higher in the pembrolizumab and placebo group was 78.0% vs. 73.0%, respectively (3). Most Serious TRAEs occurred during the neoadjuvant phase, at a rate of 32.5% vs. 19.5% in the pembrolizumab and placebo groups, with neutropenia (14.6% vs. 12.1%), anemia (2.6% vs. 2.1%) and pyrexia (2.6% vs. 0.3%) being the most common. In the neoadjuvant phase of IMpassion031, serious TRAEs occurred in 37 (23%) and 26 (16%) patients in the pembrolizumab and placebo group respectively. Grade 3–4 AEs was 103 (63%) vs. 101 (60%) in the atezolizumab and placebo group, including leucopenia [14 (9%) vs. 8 (5%)], increased aspartate aminotransferase [7 (4%) vs. 3 (2%)], and pneumonia [5 (3%) vs. 0].
The cTRIO clinical trial for early-stage TNBC patients in China
Although Chinese patients account for 18% of all breast cancer cases worldwide, relevant immunotherapy data in early-stage TNBC remain scarce. In ESMO Asia Virtual Congress 2020, the evaluated outcomes of KEYNOTE-522 among Asian patients were reported (27). Until Sep 24, 2018, 215 subjects from Korea, Japan, Taiwan (China), and Singapore were randomized 2:1 to pembrolizumab (n=136) or placebo (n=79). After a median follow-up of 13 months, pCR rates were 59% (95% CI, 47–70%) vs. 40% (95% CI, 26–55%) in the pembrolizumab and placebo groups, with a difference of 19% (95% CI, 1–35%), which is greater than the difference observed in the overall ITT populations (14%). pCR rates were also comparable between the PD-L1 positive (71% vs. 63%) and negative subgroups (51% vs. 26%). The incidence of grade ≥3 TRAEs was 75% with pembrolizumab vs. 76% with placebo, with no deaths in either group. These results demonstrated a clinically significant improvement in pCR rates in Asians with early-stage TNBC. The subgroup analysis of the IMpassion031 trial also revealed that the pCR difference between the immunotherapy and the control groups was more significant in the Asian population than in other populations (28). Correspondingly, after fully considering China’s national circumstances and the differences between Chinese and Western nations, CSCO updated the breast cancer guidelines to include several recommendations regarding neoadjuvant/adjuvant immunotherapy. Therefore, trials with Chinese patients as the primary study subjects are worth anticipating.
As a newly registered multicenter phase II trial, the cTRIO clinical trial (ChiCTR2100041675) is initiated by investigators to evaluate tislelizumab combined with nab-paclitaxel and carboplatin in neoadjuvant/ adjuvant therapy for Chinese patients with TNBC (Figure 1). Tislelizumab, specifically engineered to minimize Fcγ receptor binding to limit antibody-dependent phagocytosis, is a humanized IgG4 monoclonal antibody with a high affinity and specificity for PD-1 (29,30). Furthermore, the antitumor activity and safety of tislelizumab have been demonstrated in Chinese and other populations with solid tumor (30). In the cTRIO study, we will apply 6 cycles of TP regimen in the neoadjuvant phase, as well as sequential ICI for both neoadjuvant and adjuvant therapy. Specifically, this trial will include 62 newly diagnosed TNBCs (T1N1–3 or T2–4N0–3) with available tissue samples for PD-L1 assessment. In the neoadjuvant phase, study subjects will receive six cycles of an intravenous infusion of tislelizumab (200 mg, Q3W) in combination with chemotherapy (d1, d8, Q3w) of albumin paclitaxel (125 mg/m2) plus carboplatin AUC2, followed by a radical surgery 3–6 weeks after the final cycle of the neoadjuvant treatment. After operation, tislelizumab administration (200 mg, d1, Q3W) will be continued for 11 cycles as adjuvant monotherapy. The primary endpoint is pCR (ypT0/Tis ypN0) at the time of definitive surgery. The secondary endpoints include the pCR rate in PD-L1-positive or -negative patients, EFS, OS, and safety at one, two, and three years according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The safety and tolerability will be determined according to NCI-CTCAE v5.0. The exploratory objectives include biomarkers in tumor tissue and peripheral fibrosis associated with efficacy, drug resistance, and/or progressive disease.
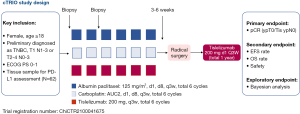
The Simon Phase 2 (Simon, 1989) design will be used to test the pCR (ypT0/Tis ypN0) efficacy of the experimental drug. An interim efficacy and safety analysis will be performed when 32 subjects are enrolled. Among 32 subjects in the first phase, if the pCR rate (ypT0/Tis ypN0) was achieved in 13 or less valid cases, the second phase was not performed. If there were more than 13 effective cases, the second phase was continued to a total of 62 subjects.
Discussion and conclusions
As can be seen, the results of current clinical trials varied considerably (Table 1). Not only did KEYNOTE-522 and IMpassion031 demonstrated significant pCR benefits for pembrolizumab and atezolizumab in combination with neoadjuvant chemotherapy regardless of PD-L1 expression, but they also demonstrated improved long-term survival in patients with early-stage TNBC (15). However, in a comparable patient population, the NeoTRIP trial with atezolizumab failed to significantly improve pCR rates when compared to chemotherapy alone, even though PD-L1 expression was associated with immunotherapy response (31). As a result, there are still numerous obstacles to overcome before neoadjuvant/adjuvant immunotherapy becomes the standard of care for early-stage TNBC.
Nevertheless, the application of immunotherapy in neoadjuvant/adjuvant treatment in early-stage TNBC is an irresistible clinical research trend. There are mainly three research directions worthing to explore clinically: (I) the first might be the optimal partner regimens of immunotherapy so as to balance efficacy benefits and adverse effects; (II) the second might be the predictive biomarkers to seek out precisely the patients who actually benefit from immunotherapy; (III) the third might be the timing to introduce immunotherapy into the early stage treatment. Will it be better in neoadjuvant, or adjuvant or neoadjuvant-guided adjuvant treatment?
The cTRIO study in China will assist in identifying more precisely which patients may benefit the most, as well as defining more precisely the optimal partner regimens that will help make the clinical benefit more robust for Chinese patients with TNBC.
Acknowledgments
Funding: This study was supported by BeiGene Co., Ltd., Beijing, China (No. BGB-A317-2005-IIT).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-4/coif). ZJ serves as the Editor-in-Chief of Translational Breast Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783-91. [Crossref] [PubMed]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010;363:1938-48. [Crossref] [PubMed]
- Barroso-Sousa R, Tolaney SM. Pembrolizumab in the preoperative setting of triple-negative breast cancer: safety and efficacy. Expert Rev Anticancer Ther 2020;20:923-30. [Crossref] [PubMed]
- Kwapisz D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol Immunother 2021;70:607-17. [Crossref] [PubMed]
- Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med 2022;386:556-67. [Crossref] [PubMed]
- Miyashita M, Ishida T. Prospect of immunotherapy in neoadjuvant/adjuvant treatment for early breast cancer. Chin Clin Oncol 2020;9:28. [Crossref] [PubMed]
- Jiang Z, Li J, Chen J, et al. Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines 2022. Transl Breast Cancer Res 2022;3:13. [Crossref]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2:361-70. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol 2019;5:74-82. [Crossref] [PubMed]
- Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014;21:15-25. [Crossref] [PubMed]
- Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014;20:1301-9. [Crossref] [PubMed]
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105-13. [Crossref] [PubMed]
- Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immuno-therapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019;25:477-86. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Nanda R, Liu MC, Yau C, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol 2020;6:676-84. [Crossref] [PubMed]
- Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020;396:1090-100. [Crossref] [PubMed]
- Gianni L, Huang CS, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol 2022;33:534-43. [Crossref] [PubMed]
- Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279-88. [Crossref] [PubMed]
- Duan J, Cui L, Zhao X, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2020;6:375-84. [Crossref] [PubMed]
- Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol 2016;17:345-56. [Crossref] [PubMed]
- Zhang P, Yin Y, Mo H, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 2016;7:60647-56. [Crossref] [PubMed]
- Gluz O, Nitz U, Liedtke C, et al. Comparison of Neoadjuvant Nab-Paclitaxel+Carboplatin vs Nab-Paclitaxel+Gemcitabine in Triple-Negative Breast Cancer: Randomized WSG-ADAPT-TN Trial Results. J Natl Cancer Inst 2018;110:628-37. [Crossref] [PubMed]
- Emens LA, Adams S, Barrios CH, et al. LBA16 IMpassion130: Final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol 2020;31:S1148. [Crossref]
- Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, place-bo-controlled, double-blind, phase 3 clinical trial. Lancet 2020;396:1817-28. [Crossref] [PubMed]
- Schmid P, Cortes J, Bergh JCS, et al. KEYNOTE-522: Phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo + chemo as neoadjuvant therapy followed by pembro vs placebo as adjuvant therapy for triple-negative breast cancer (TNBC). J Clin Oncol 2018;36:TPS602. [Crossref]
- Saji S, Mittendorf E, Harbeck N, et al. 3MO - IMpassion031: Results from a phase III study of neoadjuvant (neoadj) atezolizumab + chemo in early triple-negative breast cancer (TNBC). Ann Oncol 2020;31:S1243. [Crossref]
- Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67:1079-90. [Crossref] [PubMed]
- Dahan R, Sega E, Engelhardt J, et al. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015;28:285-95. [Crossref] [PubMed]
- Gianni L, Huang CS, Egle D, et al. Abstract GS3-04: Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res 2020;80:GS3-04-GS3.
Cite this article as: Hao X, Gao X, Yin S, Jiang Z. Prospect of neoadjuvant/adjuvant immunotherapy in early-stage triple-negative breast cancer. Transl Breast Cancer Res 2023;4:6.


