
A round table discussion: clinical landscape of trastuzumab deruxtecan in breast cancer: a retrospective and prospective view
Introduction
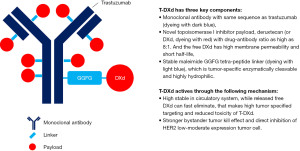
In recent decades, innovative targeted drugs have greatly advanced the treatment of breast cancer (1). A new class of targeted therapies called antibody-drug conjugates (ADCs) have begun to be explored and greatly impact the clinical landscape of cancer treatment (2), with trastuzumab deruxtecan (T-DXd, DS-8201) being one of the most successful ADC. T-DXd is a revolutionary next-generation innovative anti-HER2 drug with multifaceted properties that enhance its antitumor effect and maintain a manageable safety profile. Its humanized anti-HER2 IgG1 monoclonal antibody was developed as trastuzumab biosimilar, and each monoclonal antibody contains about 8 novel topoisomerase I inhibitor payload molecules, called DXd via stable tumor-specific cleavage tetra-peptide linker (3) (Figure 1). This design seems give the agent some advantages over previous ADCs. Firstly, the killing range was higher in HER2-expressing tumors. In preclinical trials, T-DXd can kill HER2 immunohistochemical (IHC) 1+, 2+ and 3+ cells, whereas trastuzumab emtansine (T-DM1) can only act on HER2 3+ cells (4). Secondly, the potent bystander killing effect of T-DXd is due to the characteristics of the cleavage linker and membrane permeable payload. This can kill HER2-negative tumor cells surrounding to HER2-expressing tumor cells, which will be able to act on HER2 heterogeneity (5). Third, the stable conjugation technology conferred by the hydrophobic linker, the tumor-specific lysosome-degrading linker, increases the highly selective targeting. The payload has a short half-life of 1.37 hours (6), ensuring no toxicity builds up after the payload is released. The design of the T-DXd sets a new standard for current ADC product development. There have been five publications on New England Journal of Medicine and 4 approved indications reported regarding T-DXd.
DESTINY-BREAST trial series of T-DXd have sparked a great deal of interest and attractions among experts recently (Figure 2). The DESTINY-BREAST01 is the first impressive trial with an unprecedented 19.4-month median progression-free survival (mPFS) of T-DXd for the 3rd or later line therapy of HER2-positive metastatic breast cancer (MBC) (7,8). Not long later, the DESTINY-BREAST03 made a new record for T-DXd in HER2-positve MBC, with a 25.1-month mPFS by investigator assessment (9). T-DXd has not only significantly changed the treatment of HER2-postive MBC, but will also re-define the paradigm of HER2-low breast cancer. Recently, the DESTINY-BREAST04 trial has been reported to meet its co-primary end points of progression-free survival (PFS) and overall survival (OS), regardless of hormonal receptor (HR) expression status (10,11).
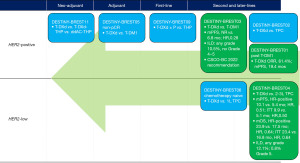
The clinical data is appealing, but the clinical practice is complicated. Whether the level II recommendation of T-DXd as treatment for patients failed to trastuzumab therapy, prior to approval in China is reasonable? And what’s the optimized sequent therapy for patients had failed to anti-HER2 TKIs and ADC? What’s the implications of T-DXd in patients with brain metastasis (BM)? What’s the expectation of T-DXd in further landscape as first-line therapy, neoadjuvant or adjuvant therapy with the ongoing trials of DESTINY-BREAST09, DESTINY-BREAST11 and DESTINY-BREAST05? For HER2-low, HR-positive patients, what’s the ideal time-window for endocrine therapy (ET) and chemotherapy (CT) switching? What’s the best indicator to evaluate the efficacy of later line therapy for HR-positive MBC patients, such as objective response (ORR), duration of response (DOR), PFS or OS? What’s will be treatment strategy for patients with HER2-low MBC? These questions are yet to be answered clearly. So, in a round table discussion, numerous breast cancer oncologists and surgeons came together to discuss the exciting advances of T-DXd and its implications in real world clinical practice. The highly summary is recorded as this review article.
T-DXd current status in clinical practice based on the success of DESTINY-BREAST03
DESTINY-BREAST03 was a phase 3 trial aim for the comparison of T-DXd with T-DM1 for the treatment of HER2-positive MBC, and was reported to meet the primary endpoint of PFS (9). The trial enrolled 524 patients with prior trastuzumab therapy; among them, 60% of the patients were also treated with pertuzumab (P). T-DXd showed significant improvement in mPFS (not reached vs. 6.8 months; hazard ratio, 0.28) and ORR (79.7% vs. 34.2%). The immature OS date also showed improvement trend (12-month survival rate, 94.1% vs. 85.9%; hazard ratio, 0.56). Interstitial lung disease (ILD) was the most concerned adverse event (AE) leading to discontinuation of T-DXd, with incidence as 10.5% in the T-DXd arm vs. 1.9% in the T-DM1. No grade 4 and 5 ILD events were reported in this trial. This indicates population with less pre-treatment or high awareness of ILD management may attributes to reduced fatal outcomes of ILD during T-DXd therapy. Notably, the strictly management strategy of ILD was taken in the DESTINY-BREAST-03 trial. T-DXd should be interrupted for any grade of suspected ILD and permanently discontinued for Grade 2 or higher level. Steroids were proactively recommended in the management of ILD, even for Grade 1 (9).
Based on these compelling results, the latest version of Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guideline [2022] (12) recommends T-DXd as the new standard of care (SOC) for patients failed from trastuzumab therapy (level of recommendation II and level of evidence 1A). T-DXd is also recommended as an optional treatment for patients who have failed anti-HER2 tyrosine kinase inhibitors (TKI) therapy (level of recommendation II and level of evidence 2A). Since pyrotinib was well used as a second line treatment for metastatic HER2-positive breast cancer, T-DXd probably will be the preferred therapy in TKI-resistant patients as soon as it is available in China.
Expert opinion
All experts agreed that DESTINY-BREAST03’s data is strong enough to suggest us T-DXd as a 2L SOC in HER2-positive MBC. Notably, mPFS and ORR were even comparable to the regimen of trastuzumab, pertuzumab and taxane in the first treatment in the CLEOPATRA trial (13). In an updated safety analysis from ASCO 2022 (14), the general safety profile of T-DXd was consistent and tolerant. T-DXd had longer therapy duration, but exposure-adjusted incidence rates (EAIRs) of T-DXd were lower than T-DXd. The incidence of ILD/pneumonitis was not increased for patient treated with T-DXd, compared with trials. This safety update with longer duration of follow-up again showed good benefit-risk profile of T-DXd in clinical usage. However, the mechanism of resistance to T-DXd is unclear. There are no data to demonstrate the optimal sequential therapy for progression on T-DXd. An interesting ideal proposed by experts is worth exploring, namely that strategies can be determined based on mechanism of resistance to T-DXd. (I) For resistance caused by HER2 degradation or loss, anti-HER2 TKIs acting inside the cell membrane may be a better choice; (II) for payload-induced resistance, switching to another ADC may be effective; (III) for cancers with altered PI3K signaling, the efficacy of TKIs is compromised, but not T-DM1, T-DM1 is a better choice given the evidence from the biomarker analysis of the EMILIA trial (15). Therefore, new ADC may be the effective therapy if the PI3K/AKT/mTOR pathway alteration can be found.
T-DXd for brain metastatic (BM) breast cancer (BCBM): stable or active BM (Figure 3)
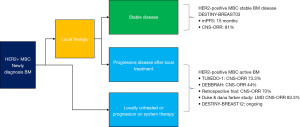
The updated results of DESTINY-BREAST03 in SABCS2021 also sparked discussions on the clinical application of T-DXd for treatment of HER2-positive MBC with central nerve system (CNS) metastasis (16). Total 36 cases were with measurable brain disease in both groups. In the T-DXd arm, 10 cases showed complete remission (CR) in BM; 17 cases’ CNS lesion had radiological reduction >30%. These data indicated that T-DXd had significant effect for the treatment of HER2-positive BM. Recently there are some clinical trials with small sample size revealed the efficacy of T-DXd in patients with active BM. Initial data from TUXEDO-1 trial showed an intracranial response rate of 73.3% (5/6), mPFS of 14 months (17). The ongoing DEBBRAH study is investigating the efficacy of T-DXd in patient who had HER2-positive or HER2-low advanced breast cancer with CNS metastasis. In breast cancer patients with active BM, the intracranial ORR for T-DXd was 44.4% (4/9) (18). Another retrospective study (19) showed that T-DXd achieved 70% CNS-ORR in 10 previously treated patients with progressive BM. More intriguingly, T-DXd also showed promising efficacy in a Duke & Dana Farber study (20) in patients with refractory leptomeningeal metastases (LM) HER2-positive breast cancer, 5/6 patients CNS-ORR was achieved. Based on those very compelling results of T-DXd efficacy for BM. The DESTINY-BREAST12 trial (NCT04739761) enrolled women with newly diagnosed or advanced BM HER2-positive MBC (21). So far, tucatinib in HER2CLIMB trial showed a promising result for active BM (22,23), and in HER2CLIMB-04 trial (NCT04539938), the combination of T-DXd and tucatinib is under testing to improve the treatment outcome of HER2-positive BCBM (24). Results from these trials will further clarify the efficacy of T-DXd in breast cancer patients with BM, especially in active BM.
Expert opinion
The efficacy of T-DXd in pretreated stable BM is very promising. However, the definition of stable or active BM is ambiguous. Experts believe that active BM is defined as symptomatic and progressive, while stable BM is considered asymptomatic or non-progressive. Clinical trials have shown that in asymptomatic patients with BM, anti-HER2 therapy without local management can have excellent outcomes (23,25). Therefore, the clinical data of T-DXd in these BM trials may be referred in clinical practice. Several trials have also explored the combination between anti-HER2 ADC & TKIs (26,27).
LM have poor prognosis. Treatment recommendations for LM are practically based on expert opinions. There has been no effective treatment option for breast cancer patients with LM (28). LM may develop with or without parenchymal disease in breast cancer patients; meanwhile, assessment of progression is based on developing new brain or meninges lesions (29,30). There has been no strong evidence regarding the treatment of anti-HER2 agents in patients with LM, either isolated or synchronous with BM. One case report had showed efficacy of anti-HER2 TKI in management of breast cancer with LM (31). At the same time, the efficacy in brain parenchyma and meninges were not assessed separately. Despite T-DXd data in HER2-positive MBC with LM from a small population, the data of CNS-ORR is promising (20). Consider the limitation of sample size, it is better to design a nationwide multicenter clinical trial to investigate this special metastatic subtype. Meanwhile, real-world studies may help us to understand the current treatment of breast cancer patients with brain and leptomeningeal disease. T-DXd as a good option is also worth exploring in larger sample sizes.
Prospect of T-DXd in HER2-positive breast cancer: ongoing studies in first line and early breast cancer (EBC)
The therapeutic landscape for HER2-positive BC in first-line, neoadjuvant and adjuvant settings has been changing rapidly (32). With the power of T-DXd, it has great confidence to step into these front lines.
DESTINY-BREAST09 (NCT04784715) is the first global trial of T-DXd in first-line MBC setting, T-DXd is being administered with or without pertuzumab comparison with the current standard of care for women with HER2-positive MBC. Results from this head-to-head phase III trial will provide answers as to whether T-DXd alone or as part of a combination regimen could provide more effective first-line HER2-directed therapy for women with HER2-positive MBC.
DESTINY-BREAST05 (NCT04622319) is a phase III, randomized, open-label study evaluating T-DXd versus T-DM1 as adjuvant therapy for high risk HER2-positive breast cancer who have not achieved pathological complete response (pCR). The primary endpoint of the trial was invasive disease-free survival (iDFS).
DESTINY-BREAST11 (NCT05113251) is a trial to assess the efficacy of T-DXd as pre-operative system therapy for patients with high-risk HER2-positive EBC. Approximately 624 patients will be randomized 1:1:1 to receive T-DXd as monotherapy or T-DXd followed by dual blockage with trastuzumab and pertuzumab plus paclitaxel (THP), or the standard regimen with dose-density doxorubicin (A) and cyclophosphamide (C) followed by trastuzumab, pertuzumab and paclitaxel (ddAC-THP). The primary endpoints will be pCR.
Another exploratory trial, DESTINY-BREAST07 (NCT04538742), is underway to explore the safety and efficacy of combination therapy of T-DXd with other anticancer drugs in HER2-positive MBC patients.
Expert opinion
Considering the big success of DESTINY-BREAST03 in second-line settings, future results of DESTINY-BREAST09 is worth looking forward to. However, most experts believe that choice of first-line therapy should be referred to activity, tolerance, and safety. The benefit of OS is associated with tolerance and patients’ compliance, longer OS comes from scenario of all lines of anti-HER2 therapy. Current data and clinical guidelines suggest that regimen with dual antibodies is still the SOC for who are sensitive to trastuzumab in first-line anti-HER2 therapy, it might not be changed until the long-term follow-up to prove that T-DXd can have great efficacy as well as good quality of life.
Currently, the combination of trastuzumab, pertuzumab and CT can achieve nearly 60% pCR in the neoadjuvant setting (33-35). The attempt with DESTINY-BREAST11 in a neoadjuvant environment is challenging. But some patients may not respond to PH therapy in the first two neoadjuvant cycles, switching to T-DXd may be an interesting endeavor worth exploring.
A new era of HER2-low breast cancer
HER2-low is an emerging breast cancer treatment concept that will be truly defined by the data released by anti-HER2 ADC (36). HER2-low accounts for 45–55% of the entire breast cancer population (37). But there are no approved anti-HER2 treatments in these patients, and those with low HER2 are considered HER2-negative. There have been several unsuccessful attempts to explore the efficacy of previous anti-HER2 drugs in HER2-low patients (38-41). T-DXd brings some breaking through data in this new era, we summarized the clinical data of T-DXd in HER2-low breast cancer in Table 1.
Table 1
| Trial | Patient | Intervention | Outcomes |
|---|---|---|---|
| J101 | HER2-low MBC, N=54 | T-DXd (5.4 or 6.4 mg/kg)a | ORR, 37%; mPFS, 10.4 mo |
| DESTINY-BREST04 | HER2-low MBC HR-positive (N=494) or HR-negative (N=63) PD on ET and CT |
T-DXd vs. TPCb | mPFS, 10.1 vs. 5.4 mo in HR-positive, hazard ratio 0.51, P<0.001; 9.9 vs. 5.1 mo in ITT, hazard ratio 0.50, P<0.001 mOS, 23.9 vs. 17.5 mo in HR-positive, hazard ratio 0.64, P=0.003; 23.4 vs. 16.8 mo in ITT, hazard ratio 0.64, P=0.001 |
| DESTINY-BREST08 (module 4 and 5) | HER2-low HR-positive MBC Module 4, N=6; module 5, N=6 |
T-DXd + anastrozole (module 4) T-DXd + fulvestrant (module 5) |
RP2D |
| DAISY | MBC Cohort 2: HER2-low, N=44 Cohort 3: HER2 IHC 0, N=44 |
T-DXd | Cohort 2: ORR, 37.5%; mPFS, 6.7 mo Cohort 3: ORR, 29.7%; mPFS, 4.2 mo |
| DS8201-A-U105 | HER2-low MBC, N=16 | T-DXd + nivolumab | ORR, 50%, mPFS,7.0 mo, OS, 19.5 mo |
| BEGONIA arm 6 | HER2-low mTNBC, no pre-CT, N=21 | T-DXd + durvalumab (arm 6) | ORR, 66.7% |
| DESTINY-BREST06 | HER2-lowc HR-positive MBC PD on ET and no pre-CT, N=850 |
T-DXd vs. TPCd | PFS and OS, not reported |
| TALENT | HER2-low HR-positive EBC, N=58 | T-DXd ± anastrozole as neoadjuvant | pCR, not reported |
a, T-DXd is administrated at dosage of 5.4 mg/kg q3w, else other specified; b, treatment of physician’ choice in DB04 includes capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel; c, HER2 IHC >0 and <1+ is included; d, treatment of physician’ s choice in DB06 includes capecitabine, paclitaxel, or nab-paclitaxel. MBC, metastatic breast cancer; T-DXd, trastuzumab deruxtecan; ORR, objective response rate; mPFS, median progression-free survival; mo, month; HR, hormonal receptor; PD, progression disease; ET, endocrine therapy; CT, chemotherapy; TPC, treatment of physician’s choice; mOS, median overall survival; ITT, intention-to-treatment population; RP2D, recommended dose of phase two trial; IHC, immunohistochemical; OS, overall survival; mTNBC, metastatic triple-negative breast cancer; EBC, early breast cancer, pCR, pathological complete response.
J101, a phase Ib study evaluating the safety and efficacy of T-DXd in HER2-low MBC, demonstrated an ORR of 37.0% for T-DXd. The DOR was 10.4 months in heavily pretreated patients. Currently, the HER2-low population was explored in two phase III trials (42).
The DESTINY-BREAST04 trial (10) investigated the efficacy and safety of T-DXd compared with CT by physician’s choice (TPC) for the treatment of advanced breast cancer with HER2-low expression. Four hundred and eighty HR-positive and 60 HR-negative HER2-low (HER2 IHC 1+ or HER2 IHC 2+/ISH−) were registered. Patients should receive one or two lines of CT, at least one line of ET, and be assessed by the investigator as not benefiting from ET if HR-positive. As reported, both PFS and OS in T-DXd arm were statistically significant and clinically meaningful improved compared with TPC arm. The efficacy was consistent in either HR-positive or HR-negative or whole population. The mPFS in HR-positive and all patients were 10.1 months in T-DXd arm versus 5.4 months in TPC arm, respectively (HR, 0.51; P<0.001) and 9.9 months versus 5.1 months in each arm respectively (HR, 0.50; P<0.001). The median OS in HR-positive and all patients were 23.9 months in T-DXd arm and 17.5 months in TPC arm, respectively (HR, 0.64; P=0.003) and 23.4 months versus 16.8 months in each arm, respectively (HR, 0.64; P=0.001).
Interestingly, a report from an exploratory DAISY trial presents a new concept of HER2 ultra-low (43). One hundred and seventy-nine pre-treated patients were recruited into the trial, all of whom were assigned to one of three cohorts based on HER2 expression. HER2 overexpression (n=68), defined as HER2 immunohistochemically (IHC) score of 3+ or IHC 2+ and positive in situ hybridization; HER2-low (n=73), defined as HER2 IHC 2+ and negative ISH result or IHC 1+; or HER2-negative (n=38), defined as HER2 IHC 0. T-DXd treatment achieved ORR rate of 71.0% in the HER2-overexpressing cohort, 37.5% in the HER2-low cohort, and 30.0% in the HER2-negative cohort. This is the first trial to show that T-DXd may be active in patients with ultra-low HER2 expression.
Concurrently, the DESTINYBreast06 (NCT04494425) trial is evaluating the comparison between T-DXd versus physician-chosen CT in patients with HER2-low, HR-positive breast cancer with at least two lines of previous ET, or fast progression on CDK4/6 inhibitors in the metastatic setting within 6 months. Patients should not receive CT in the metastatic setting. The trial plans to enroll approximately 700 HER2-low (IHC1+ or IHC2+/ISH−) and 150 HER2 ultra-low (HER2 IHC >0 and <1+) patients. The results of this study may provide evidence to support T-DXd to be a new option for patients with advanced HER2-low breast cancer who are refractory to ET. And could address the benefit of T-DXd in patients with ultra-low HER2 as well.
An exploratory trial, DESTINY-BREAST08 (NCT04556773), is also underway to investigate the safety and efficacy of combination therapy with T-DXd and other agents in MBC with HER2-low expression.
With the data shown above, the T-DXd-targeted population will go beyond the limits of HER2 positivity, ushering in a new era of HER2-low.
Expert opinion
The testing of HER2-low is a big challenge, and it is difficult to differentiate between 0 and 1+ HER2 ICH scores (44). While new approaches to address these issues are being explored, including AI imaging (45), HER2 mRNA (46), and more. HER2 heterogeneous is another issue for precise HER2-low identification. For an example, HER2 re-testing for 2 formalin-fixed paraffin-embedded blocks in a patients may be read as different scores. It’s worth exploration that HER2-low diagnostic rate may increase if scoring 2 blocks in a HER2 testing, and then this may make more patients benefit from treatment with T-DXd. More evidence is needed on whether HER2 ultra-low can benefit from T-DXd, considering available HER2 monoclonal antibodies currently used to test for HER2 antigen are less sensitive. While IHC detection is a semi-quantitative method, a true quantitative method may provide more evidence. More real-world data on treatment patterns and efficacy are needed to elucidate the position of T-DXd after its approval in HER2-low breast cancer. Combination of T-DXd with other anti-cancer therapy in HER2-low was another interest topic discussed. In the HR-positive and HER2-low expression breast cancer, combination of T-DXd and ET may be effective. The DESTINY-BREAST08 study (47) reported on ASCO annual meeting 2022 showed that no dose-limited toxicity (DLT) was reported for T-DXd (5.4 mg/kg q3w) combination with anastrozole (1 mg daily) or fulvestrant [500 mg every 4 weeks (loading dose: 500 mg cycle 1 days 1 and 15)], but no efficacy data was reported this time and the study is under further follow-up. As DAISY trial (48) has proved that the efficacy of T-DXd is associated with level of HER2 expression. HER2-low patients who may have less PFS or ORR compared to that in HER2-positive patients, may have additional benefit from T-DXd combinations. More radically T-DXd as neoadjuvant in HER2-low EBC is also could be considered, since the pCR rate in HR-positive EBC is relatively lower. A trial has been reported to explore for T-DXd with or without anastrozole as neoadjuvant treatment (49).
The clinical positioning of T-DXd in HER2-low HR-positive MBC has be more clarified with the data from DESTINY-BREAST04. The HER2-low HR-positive metastatic patients have been treated as HER2-negative patients and normally will start CDK4/6 inhibitor as first or second-line therapy and then receive PI3K or mTOR inhibitors plus ET. When the patients are refractory to ET, one or two lines of CT will also be administrated, which include platinum-based regimen, or taxane or capecitabine mono therapy. After that T-DXd may be administrated. There will also be another ADC, sacituzumab govitecan has been on the scientific meetings this year in HR-positive MBC (50). So, the sequencing between these two ADC is also very interesting to be discussed.
Acknowledgments
We would like to thank Mr. Yuanqing Gao for his support on preparing this review article.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Corti C, Giugliano F, Nicolò E, et al. Antibody-Drug Conjugates for the Treatment of Breast Cancer. Cancers (Basel) 2021;13:2898. [Crossref] [PubMed]
- Tarantino P, Carmagnani Pestana R, Corti C, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin 2022;72:165-82. [Crossref] [PubMed]
- Yver A, Agatsuma T, Soria JC. The art of innovation: clinical development of trastuzumab deruxtecan and redefining how antibody-drug conjugates target HER2-positive cancers. Ann Oncol 2020;31:430-4. [Crossref] [PubMed]
- Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res 2016;22:5097-108. [Crossref] [PubMed]
- Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107:1039-46. [Crossref] [PubMed]
- Nagai Y, Oitate M, Shiozawa H, et al. Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica 2019;49:1086-96. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
- Krop IE, Saura C, Yamashita T, et al. Abstract GS1-03: [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) in subjects with HER2-positive metastatic breast cancer previously treated with T-DM1: A phase 2, multicenter, open-label study (DESTINY-Breast01). Cancer Res 2020;80:GS1-03. [Crossref]
- Cortés J, Kim SB, Chung WP, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 2022;386:1143-54. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Results of DESTINY-Breast04, a randomized, phase 3 study. J Clin Oncol 2022;40:abstr LBA3.
- Jiang Z, Li J, Chen J, et al. Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines 2022. Transl Breast Cancer Res 2022;3:13. [Crossref]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Hamilton EP, Bragaia VPH, Yeo W, et al. Trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in patients (pts) with HER2-positive (HER2+) unresectable and/or metastatic breast cancer (mBC): Safety follow-up of the randomized, phase 3 study DESTINY-Breast03. J Clin Oncol 2022;40:1000. [Crossref]
- Baselga J, Lewis Phillips GD, Verma S, et al. Relationship between Tumor Biomarkers and Efficacy in EMILIA, a Phase III Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer. Clin Cancer Res 2016;22:3755-63. [Crossref] [PubMed]
- Hurvitz S, Kim S-B, Chung W-P, et al. Abstract GS3-01: Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. Cancer Res 2022;82:GS3-01. [Crossref]
- Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 2022;28:1840-7. [Crossref] [PubMed]
- Pérez-García JM, Batista MV, Cortez P, et al. Trastuzumab Deruxtecan in Patients with Central Nervous System Involvement from HER2-Positive Breast Cancer: The DEBBRAH Trial. Neuro Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Kabraji S, Ni J, Sammons S, et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res 2022; Epub ahead of print. [Crossref] [PubMed]
- Alder L, Trapani D, Van Swearingen A, et al. Abstract 5257: Durable clinical and radiographic responses in a series of patients with HER2+ Breast Cancer (BC) Leptomeningeal Disease (LMD) treated with trastuzumab deruxtecan (T-DXd). Cancer Res 2022;82:5257. [Crossref]
- Lin NU, Ciruelos E, Jerusalem G, et al. Abstract OT2-26-01: Open-label, multinational, multicenter, phase 3b/4 study of trastuzumab deruxtecan (T-DXd) in patients with or without baseline brain metastasis with previously treated advanced/metastatic human epidermal growth factor receptor 2-positive breast cancer (HER2+ BC): DESTINY-Breast12. Cancer Res 2022;82:OT2-26-01.
- Murthy RK, Loi S, Okines A, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 2020;382:597-609. [Crossref] [PubMed]
- Lin NU, Borges V, Anders C, et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol 2020;38:2610-9. [Crossref] [PubMed]
- Krop IE, Ramos J, Zhang C, et al. HER2CLIMB-04: Phase 2 open label trial of tucatinib plus trastuzumab deruxtecan in patients with HER2 unresectable locally advanced or metastatic breast cancer with and without brain metastases (trial in progress). Cancer Res 2021;39:TPS1097.
- Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol 2022;23:353-61. [Crossref] [PubMed]
- Borges VF, Ferrario C, Aucoin N, et al. Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol 2018;4:1214-20. [Crossref] [PubMed]
- Abraham J, Montero AJ, Jankowitz RC, et al. Safety and Efficacy of T-DM1 Plus Neratinib in Patients With Metastatic HER2-Positive Breast Cancer: NSABP Foundation Trial FB-10. J Clin Oncol 2019;37:2601-9. [Crossref] [PubMed]
- Pellerino A, Internò V, Mo F, et al. Management of Brain and Leptomeningeal Metastases from Breast Cancer. Int J Mol Sci 2020;21:8534. [Crossref] [PubMed]
- Figura NB, Rizk VT, Armaghani AJ, et al. Breast leptomeningeal disease: a review of current practices and updates on management. Breast Cancer Res Treat 2019;177:277-94. [Crossref] [PubMed]
- Abouharb S, Ensor J, Loghin ME, et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat 2014;146:477-86. [Crossref] [PubMed]
- Yan F, Rinn KJ, Kullnat JA, et al. Response of Leptomeningeal Metastasis of Breast Cancer With a HER2/neu Activating Variant to Tucatinib: A Case Report. J Natl Compr Canc Netw 2022;20:745-52. [Crossref] [PubMed]
- Yan Y, Li Q, Li J. Round table discussion: strategies for the treatment of HER2-positive advanced breast cancer in the rising age of antibody-drug conjugates. Transl Breast Cancer Res 2022;3:18. [Crossref]
- Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2018;19:115-26. [Crossref] [PubMed]
- Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol 2018;29:646-53. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [Crossref] [PubMed]
- Marchiò C, Annaratone L, Marques A, et al. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 2021;72:123-35. [Crossref] [PubMed]
- Tarantino P, Hamilton E, Tolaney SM, et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol 2020;38:1951-62. [Crossref] [PubMed]
- Fehrenbacher L, Cecchini RS, Geyer CE Jr, et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J Clin Oncol 2020;38:444-53. [Crossref] [PubMed]
- Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res 2008;14:7861-70. [Crossref] [PubMed]
- Burris HA 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011;29:398-405. [Crossref] [PubMed]
- Gianni L, Lladó A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010;28:1131-7. [Crossref] [PubMed]
- Modi S, Park H, Murthy RK, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 2020;38:1887-96. [Crossref] [PubMed]
- Venetis K, Crimini E, Sajjadi E, et al. HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer. Front Mol Biosci 2022;9:834651. [Crossref] [PubMed]
- Fernandez AI, Liu M, Bellizzi A, et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol 2022;8:1-4. [Crossref] [PubMed]
- Gustavson M, Haneder S, Spitzmueller A, et al. Abstract PD6-01: Novel approach to HER2 quantification: Digital pathology coupled with AI-based image and data analysis delivers objective and quantitative HER2 expression analysis for enrichment of responders to trastuzumab deruxtecan (T-DXd; DS-8201), specifically in HER2-low patients. Cancer Res 2021;81:PD6-01. [Crossref]
- Eiger D, Agostinetto E, Saúde-Conde R, et al. The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers (Basel) 2021;13:1015. [Crossref] [PubMed]
- Andre F, Hamilton EP, Loi S, et al. Dose-finding and -expansion studies of trastuzumab deruxtecan in combination with other anti-cancer agents in patients (pts) with advanced/metastatic HER2+ (DESTINY-Breast07 [DB-07]) and HER2-low (DESTINY-Breast08 [DB-08]) breast cancer (BC). J Clin Oncol 2022;40:3025. [Crossref]
- Diéras V, Deluche E, Lusque A, et al. Abstract PD8-02: Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY). Cancer Res 2022;82:PD8-02. [Crossref]
- Hurvitz SA, Peddi PF, Tetef ML, et al. TRIO-US B-12 TALENT: Phase II neoadjuvant trial evaluating trastuzumab deruxtecan with or without anastrozole for HER2-low, HR early stage breast cancer. Cancer Res 2021;39:TPS603.
- Rugo HS, Bardia A, Marmé F, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol 2022;40:3365-76. [Crossref] [PubMed]
Cite this article as: Liang X, Yan Y, Song G. A round table discussion: clinical landscape of trastuzumab deruxtecan in breast cancer: a retrospective and prospective view. Transl Breast Cancer Res 2022;3:32.


