
Risk-reducing surgery in PALB2 mutations carriers with breast cancer: a case series and literature review
Introduction
While most breast cancer arises de novo, approximately 10% of breast cancer cases are due to inherited genetic mutations (1). Breast cancer with a genetic predisposition is most commonly associated with mutations in the BReast CAncer gene 1 (BRCA1) and BReast CAncer gene 2 (BRCA2) (2). More recently, numerous other mutations including partner and localizer of BRCA2 (PALB2), cell cycle checkpoint kinase 2 (CHEK2), and ataxia-telangiectasia mutated (ATM) have shown an increased risk for breast cancer development especially when combined with other factors such as personal and family history (3). A recent nationwide study in the United States found that out of 58,798 women diagnosed with breast cancer, 0.91% were found to have germline PALB2 mutations (1). Despite recognition of PALB2 as an important breast cancer gene, there is currently a dearth of guidelines on the clinical management of PALB2 mutation carriers with breast cancer (4).
An international case-control study of 34 known or suspected breast cancer susceptibility genes found that protein-truncating variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2 were significantly associated with a risk of breast cancer and categorized variants in BRCA1, BRCA2, and PALB2 as high-risk based on estimated absolute risks of breast cancer exceeding 30% by age 80 years (5). An analysis of the population-based studies in the Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium showed that pathogenic PALB2 variants were associated with a moderate risk of breast cancer and identified PALB2 as a high-risk gene among patients with a family history of breast cancer (6). PALB2 pathogenic variants confer an estimated lifetime absolute risk of breast cancer of 33–58% and increase the risk of triple negative breast cancer, ovarian cancer, pancreatic cancer, and male breast cancer (6-9). When factoring in polygenic risk score (PRS), mammographic density, hormonal exposure and lifestyle risk factors, nearly a third of PALB2 mutation carriers would have BRCA1/BRCA2 equivalent lifetime breast cancer risks of greater than 60% (4). The independently modifying effect of PRS is combined with family history and other conventional risk factors into a personalized risk assessment for each patient.
With the development and expansion of multigene panel testing, pathogenic variants in genes such as PALB2 are now detected more often. This has led to the development of gene specific screening guidelines (10). The National Comprehensive Cancer Network (NCCN) recommendations for screening patients with BRCA1, BRCA2 and PALB2 as well as other high and moderate penetrance mutations are well established. They include increased breast cancer screening beginning at age 30 years with annual mammograms and breast magnetic resonance imaging (MRI) with contrast (11). In addition to active surveillance, a patient with a PALB2 pathogenic variant may elect to undergo risk-reducing surgery (12). The indications for risk-reducing surgery in the form of prophylactic mastectomy in PALB2 mutation carriers are unclear in the current literature. However, a retrospective analysis by Bergstrom et al. found that patients with breast cancer and moderate penetrance gene mutations (ATM, CHEK2, or PALB2) were more likely to undergo a total mastectomy and contralateral risk-reducing mastectomy (CRRM) than those without mutations (13).
Our study is a retrospective, single-center case series of five patients with PALB2 pathogenic variants who underwent surgical management of their breast cancer between February 2020 and August 2022 at an academic institution in Washington, DC. We will discuss the role of CRRM in breast cancer patients with germline PALB2 mutations. We present the following article in accordance with the AME Case Series reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-33/rc).
Methods
In this retrospective case series, we present 5 patients with PALB2 mutations between the ages of 29 and 61 years who were diagnosed with breast cancer. The patients underwent surgical management of their breast cancer at our urban academic institution in Washington, DC between November 2020 and March 2022. Through their clinical courses and a literature review, we discuss the role of CRRM in breast cancer patients with PALB2 gene mutations.
Case #1
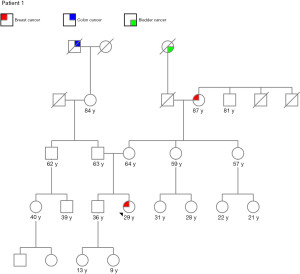
A 29-year-old Gravida (G) 0 Para (P) 0 African-American female presented after noting a right breast mass. Family history was positive for breast cancer in her maternal grandmother diagnosed at age 48 years, colon cancer in her paternal great grandfather, and bladder cancer in her maternal great grandmother (Figure 1). Patient had a history of contraception use in the past. Right breast ultrasound showed a 1.8 cm mass. Biopsy revealed grade 2 infiltrating mammary carcinoma with ductal and lobular features and surrounding ductal carcinoma in situ. The tumor was estrogen receptor (ER) positive, progesterone receptor (PR) positive, and human epidermal growth factor receptor 2 (HER2) negative and the Ki-67 proliferation index was 19%. An additional 4 cm linear region in the right breast seen on MRI was biopsied and found to be invasive ductal carcinoma (Figure 2).
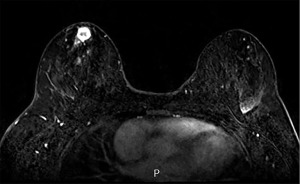
Genetic testing performed one month after initial diagnosis showed that the patient was positive for a PALB2 pathogenic variant (Table 1). Three months after initial diagnosis, the patient underwent right skin-sparing mastectomy, right axillary sentinel lymph node biopsy, risk-reducing left nipple-sparing mastectomy (NSM), and immediate bilateral tissue expander reconstruction. Surgical pathology revealed right invasive ductal carcinoma with extensive lymphovascular invasion and 1 out of 4 lymph nodes was positive. Post-operatively, the patient underwent adjuvant chemotherapy with doxorubicin, cyclophosphamide and taxol (Table 2), radiation, and endocrine therapy with an aromatase inhibitor and ovarian suppression. The patient has been followed for the last 8 months with no new recurrences or complications.
Table 1
| Case | Gene | Variant | Zygosity | Classification |
|---|---|---|---|---|
| 1 | PALB2 | c.2257C.T (p.Arg753*) | Heterozygous | Pathogenic |
| NTHL1 | c.212C>T (p.Ser71Leu) | Heterozygous | VUS | |
| 2 | PALB2 | c.3323del (p.Tyr1108Serfs*16) | Heterozygous | Pathogenic |
| MSH3 | c.421T>C (p.Cys141Arg) | Heterozygous | VUS | |
| 3 | PALB2 | c.3113G>A (p.Trp1038*) | Heterozygous | Pathogenic |
| 4 | PALB2 | c.509_510del (p.Arg170Ilefs*14) | Heterozygous | Pathogenic |
| 5 | PALB2 | Deletion (exon 11) | Heterozygous | Pathogenic |
| ATM | c.1380G>A (Silent) | Heterozygous | VUS |
VUS, variant of uncertain significance.
Table 2
| Case | Intent | Regimen name | Regimen description | Duration |
|---|---|---|---|---|
| 1 | Adjuvant | ddAC + weekly paclitaxel | Doxorubicin 60 mg/m2 IV cycles 1–4, every 14 days given on day 1 Cyclophosphamide 600 mg/m2 IV cycles 1–4, every 14 days given on day 1 Paclitaxel 80 mg/m2 IV cycles 5–8, every 21 days on days 1, 8, and 15 |
ddAC every 2 weeks for 4 weeks followed by taxol every 2 weeks for 4 weeks |
| 2 | Neoadjuvant | ddAC + dose-dense paclitaxel | Doxorubicin 60 mg/m2 IV cycles 1–4, every 14 days given on day 1 Cyclophosphamide 600 mg/m2 IV cycles 1–4, every 14 days given on day 1 Paclitaxel 175 mg/m2 IV cycles 5–8, every 14 days on day 1 |
ddAC every 2 weeks for 4 weeks followed by taxol every 2 weeks for 4 weeks |
| Adjuvant | Capecitabine | Capecitabine 1250 mg/m2 PO twice a day on days 1–14, every 3 weeks | 6 cycles | |
| 3 | Neoadjuvant | ddAC + dose-dense paclitaxel | Doxorubicin 60 mg/m2 IV cycles 1–4, every 14 days given on day 1 Cyclophosphamide 600 mg/m2 IV cycles 1–4, every 14 days given on day 1 Paclitaxel 175 mg/m2 IV cycles 5–8, every 14 days on day 1 |
ddAC every 2 weeks for 4 weeks followed by taxol every 2 weeks for 4 weeks |
| Adjuvant | Abemaciclib | Abemaciclib 150 mg PO twice a day | 2 years | |
| 4 | Neoadjuvant | ddAC + dose-dense paclitaxel | Doxorubicin 60 mg/m2 IV cycles 1–4, every 14 days given on day 1 Cyclophosphamide 600 mg/m2 IV cycles 1–4, every 14 days given on day 1 Paclitaxel 175 mg/m2 IV cycles 5–8, every 14 days on day 1 |
ddAC every 2 weeks for 4 weeks followed by taxol every 2 weeks for 4 weeks |
| 5 | Neoadjuvant | ddAC + weekly paclitaxel | Doxorubicin 60 mg/m2 IV cycles 1–4, every 14 days given on day 1 Cyclophosphamide 600 mg/m2 IV cycles 1–4, every 14 days given on day 1 Paclitaxel 80 mg/m2 IV cycles 5–8, every 21 days on days 1, 8, and 15 |
ddAC every 2 weeks for 4 weeks followed by taxol every 2 weeks for 4 weeks |
ddAC, dose-dense doxorubicin and cyclophosphamide.
Case #2
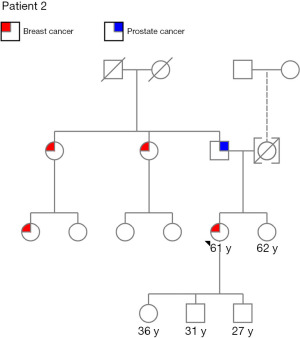
A 61-year-old G3P3 postmenopausal African-American female presented with a right breast mass. Her past medical history is significant for asthma, hypertension, hyperlipidemia, type 2 diabetes mellitus and schizophrenia. Family history is positive for prostate cancer in her father and breast cancer in two paternal aunts and a paternal first cousin (Figure 3). Patient has a 20-year history of prior oral contraceptive use. She was also an active tobacco user with 40 pack-year history of smoking. Mammogram revealed a 2.3 cm right breast mass (Figure 4) and biopsy showed poorly differentiated, grade 3 invasive ductal carcinoma that was ER negative, PR negative and HER2 negative. She received neoadjuvant chemotherapy comprised of doxorubicin, cyclophosphamide and taxol (Table 2).
About 9 months after completing neoadjuvant therapy, the patient underwent right breast lumpectomy and right axillary sentinel lymph node biopsy. Surgical pathology confirmed invasive ductal carcinoma with negative margins and no lymph nodes identified. The patient then underwent complete axillary dissection and 14 lymph nodes were removed, all of which were negative for malignancy. She received adjuvant radiation and was subsequently enrolled in a clinical trial and randomized to receive adjuvant capecitabine.
Genetic testing performed approximately one year after initial diagnosis was positive for PALB2 pathogenic variant and MSH3 variant of uncertain significance (Table 1). Risk-reducing bilateral mastectomy and oophorectomy were discussed with the patient and her family. She elected not to have risk-reducing surgery and the decision was made to continue with close surveillance. She was referred to gynecology for ovarian cancer screening and gastroenterology follow up for pancreatic and colon cancer screening. Surveillance imaging showed no evidence of malignancy (Figure 5) and she is doing well on clinic follow up with her oncologist 34 months after initial diagnosis.
Case #3
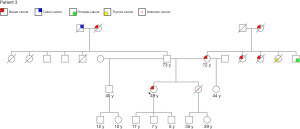
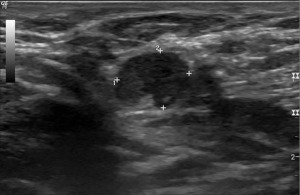
A 49-year-old G3P3 premenopausal African-American, Native American and Caucasian ancestry woman presented with a 1.4 cm left breast nodule found on screening mammogram. Her family history is positive for breast cancer in her mother diagnosed at age 65 years, two maternal aunts, and paternal grandmother, unknown cancer in her sister, thyroid cancer in her maternal aunt, prostate cancer in her maternal uncle, and colon cancer in her paternal grandfather (Figure 6). Diagnostic imaging revealed a 1.1 cm irregular nodule in addition to a 0.5 mm satellite lesion and axillary lymphadenopathy (Figure 7). Biopsy demonstrated grade 2 invasive ductal carcinoma that was ER positive, PR negative, and HER2 negative with a Ki-67 of 50% and metastatic invasive ductal carcinoma of the left axillary node. She was started on neoadjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel (Table 2).
Genetic testing performed one month after initial diagnosis was positive for a PALB2 pathogenic variant (Table 1). Approximately 7 months after initial diagnosis, the patient underwent bilateral skin-sparing mastectomy, left axillary lymph node dissection, and immediate bilateral tissue expander reconstruction. Surgical pathology revealed residual grade 2 invasive ductal carcinoma with negative margins and 5 out of 12 axillary lymph nodes positive for metastatic carcinoma. Her clinical course was complicated by right breast wound dehiscence at the T-junction with exposure of the right breast tissue expander requiring oral antibiotics, removal of her right breast tissue expander, and secondary closure by her plastic surgeon.
The patient has completed adjuvant radiation therapy and is currently doing well with no evidence of recurrence. She has been referred to gynecologic oncology given her increased risk of ovarian cancer and she plans to undergo laparoscopic bilateral salpingo-oophorectomy.
Case #4
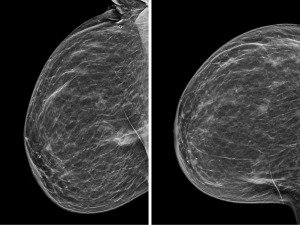
A 58-year-old G2P2 post-menopausal Caucasian female presented with a palpable left breast mass. She has family history of a maternal aunt with breast cancer and maternal grandfather with prostate cancer. Patient was also a former smoker. Diagnostic mammogram revealed a 4.5 cm irregular left breast mass, calcifications, 1.7 cm satellite lesion, and an enlarged 3.9 cm left axillary lymph node (Figure 8). Biopsy demonstrated grade 2 invasive ductal carcinoma that was ER positive, PR positive, and HER2 negative with a Ki-67 of 30%, and metastatic invasive ductal carcinoma of the axillary lymph node. Biopsy of the calcifications revealed lobular carcinoma in situ (LCIS).
Metastatic workup showed no evidence of distant metastatic disease. She underwent neoadjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel (Table 2). Genetic testing performed two months after initial diagnosis demonstrated a PALB2 pathogenic variant (Table 1). Surgical options were discussed with the patient and she leaned towards breast conservation therapy. She was referred to gynecologic oncology to discuss risk-reducing bilateral salpingo-oophorectomy but the patient deferred at the time.
About 2 months after completing neoadjuvant therapy, the patient underwent left partial mastectomy, left axillary lymph node dissection, and left breast oncoplastic reconstruction. Surgical pathology showed invasive ductal carcinoma with negative margins and metastatic ductal carcinoma in 1 out of 31 axillary lymph nodes. She completed adjuvant radiation therapy and is currently receiving adjuvant endocrine therapy with letrozole.
Case #5
A 54-year-old postmenopausal G2P1 African American female presented with a right breast cancer. Her past surgeries include a hysterectomy and family history is significant for breast cancer in a paternal half-sister diagnosed in her 40s and maternal cousin diagnosed in her 50s, an unspecified cancer in her father, liver cancer in a maternal aunt, and brain cancer in a maternal uncle. Diagnostic imaging showed a mass measuring 2.8 cm and right axillary lymphadenopathy. Biopsy demonstrated DCIS and moderately differentiated invasive ductal carcinoma that was ER, PR, and HER2 receptor negative with a Ki-67 of 15%. Bilateral axillary lymph nodes were negative for carcinoma.
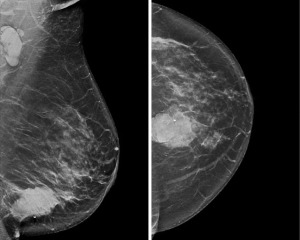
Her metastatic workup was negative. She was started on neoadjuvant chemotherapy with adriamycin, cyclophosphamide and taxol (Table 2). Post-treatment MRI demonstrated worsening disease including increased size of the mass and a new suspicious mass 4 cm from the index cancer (Figure 9). Genetic testing performed just under 8 months after initial diagnosis was positive for a PALB2 pathogenic variant as well as a variant of uncertain significance in the ATM gene (Table 1). Given these results, the patient elected for right mastectomy and CRRM.

About 8 months after initial diagnosis, the patient underwent bilateral NSM with right axillary sentinel lymph node biopsy and direct-to-implant reconstruction. Surgical pathology demonstrated two tumor beds with residual invasive ductal carcinoma and a single lymph node with isolated tumor cells. Postoperatively, the patient did well with no complications. The case series conformed to the provisions of the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this study and accompanying images. Copies of the written consents are available for review by the editorial office of this journal.
Results
A summary of cases can be seen in Table 3. The average age of patients in this series is 50.2 years. The average tumor size is 2.5 cm. Out of the 5 patients, 3 underwent CRRM and 2 patients underwent unilateral surgery for their breast cancer and chose observation for the contralateral breast. Of the 3 patients who underwent CRRM, one patient experienced wound dehiscence, a surgical complication after reconstruction of the prophylactic side that required reoperation. None of the patients developed recurrence of their breast cancer with an average length of follow up of 15.4 months.
Table 3
| Case | Age, years | Diagnosis | Size (cm) | Genetic mutation | Primary surgery | Contralateral surgery | Chemotherapy | Oophorectomy | Radiation | Endocrine | Complications | Recurrence | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | R IDC | 1.8 | PALB2 | SSM | Prophylactic NSM | Adjuvant AC + T | No | Adjuvant | AI + OS | None | None | 8 |
| 2 | 61 | R IDC | 2.3 | PALB2, MSH3 | Lumpectomy | None | Neoadjuvant AC + T, adjuvant capecitabine | No | Adjuvant | None | None | None | 34 |
| 3 | 49 | L IDC | 1.1 | PALB2 | SSM | Prophylactic SSM | Neoadjuvant DCP, adjuvant abemaciclib | Planned | Adjuvant | AI + OS | Wound dehiscence | None | 10 |
| 4 | 58 | L IDC | 4.5 | PALB2 | Lumpectomy | None | Neoadjuvant DCP | No | Adjuvant | AI | None | None | 10 |
| 5 | 54 | R IDC | 2.8 | PALB2, ATM# | NSM | Prophylactic NSM | Neoadjuvant AC + T | No | None | None | None | None | 15 |
#, variant of uncertain significances. R IDC, right invasive ductal carcinoma; L IDC, left invasive ductal carcinoma; SSM, skin sparing mastectomy; NSM, nipple sparing mastectomy; AC + T, adriamycin, cytoxan and taxol; DCP, doxorubicin, cyclophosphamide, paclitaxel; AI, aromatase inhibitor; OS, ovarian suppression.
Discussion
Compared to the general population, women with germline PALB2 mutations have nearly a fivefold increase in breast cancer risk and an estimated cumulative lifetime risk of breast cancer development of up to 58% with positive family history (12,14). Yang et al. found that inherited PALB2 pathogenic variants were significantly associated with a relative female breast cancer risk of 7.18, ovarian cancer risk of 2.91, pancreatic cancer risk of 2.37, and male breast cancer risk of 7.34 (8). In a Finnish study, breast cancer patients with PALB2 mutations were found to be associated with aggressive tumor phenotypes, higher tumor grades, higher rates of triple negative receptor status, and increased Ki-67 (15). Additionally, patients harboring ATM, CHEK2 or PALB2 gene mutations without knowledge of mutation status prior to surgery have been shown to have an increased risk of recurrence with a locoregional and distant recurrence rate of 32% as opposed to 8.5% in patients negative for mutations (13).
With evidence of up to a 90% risk reduction in the incidence of breast cancer among BRCA1 and BRCA2 mutation carriers following risk-reducing surgery, patients with increased genetic risk of breast cancer are increasingly choosing mastectomy as a cancer risk reduction strategy (16). In a 2019 study following 6,223 women with a BRCA1 or BRCA2 mutation, approximately 28% of women opted for prophylactic bilateral mastectomy (17). Although risk reduction has been found in BRCA patients, there is no significant difference in overall survival (16). In a study of 235 patients, the rate of CRRM for patients with a PALB2 mutation and unilateral breast cancer was found to be 58% (18). In BRCA patients, the cumulative risk of CBC 20 years after breast cancer diagnosis has been estimated at 40% for BRCA1 carriers and 26% for BRCA2 carriers, which likely represents the upper range of CBC risk in women with PALB2 mutations (19). In a prospective study of breast cancer patients from Poland who underwent genetic testing, CBC was reported in 10% of PALB2 mutation carriers (20). Further studies are required to determine if there is a survival benefit associated with CRRM in this patient population.
Of importance is the discussion with breast cancer patients of the inherent risk of undergoing risk-reducing mastectomy. In our case series, patient #3 experienced a surgical complication of reconstruction on her prophylactic side requiring antibiotics and reoperation. Barton et al. studied 269 women aged 18–80 years undergoing bilateral risk-reducing mastectomy without a personal history of breast cancer. Of their cohort, the complication rates were 17% infection, 17% seroma, 8% flap necrosis, 7% capsular contraction, and 11.9% systemic complications (18). Additionally, mastectomy does not eliminate the risk of developing breast cancer with a residual risk of about 5% related to the presence of residual glandular tissue or ectopic breast tissue (21). Contralateral risks, which include not only surgical complications such as skin flap necrosis, infection, and bleeding but also potential delays in adjuvant therapy, should be discussed with patients to make an informed decision.
In breast cancer patients with PALB2 mutations who choose not to undergo CRRM, active surveillance with regular follow up is recommended. After reviewing the risks and benefits, two patients in our case series decided to continue with surveillance and remain without evidence of disease on surveillance imaging. Both patients who underwent breast-conserving surgery and chose surveillance over risk-reducing surgery were notably above the age of 55 and therefore have shorter residual life expectancy and lower anticipated risk of PALB2-associated CBC (22). Screening of the contralateral breast typically consists of annual breast imaging using a combination of mammograms and breast MRI. Lowry et al. reported that breast cancer screening in patients with PALB2 pathogenic variants with both mammography and breast MRI halves breast cancer mortality (23). These mortality benefits from screening are similar to those reported in BRCA mutation carriers and should inform practice guidelines.
NSM is an oncologically safe procedure with estimated disease-free survival rates of 95.7% and 92.3% at 3 and 5 years, respectively (24). In a single institutional study of 322 patients undergoing 588 nipple-sparing mastectomies, the recurrence rate was 3.1% (25). Risk-reducing NSM has been shown to be an oncologically safe surgical option resulting in significant breast cancer reduction in a BRCA population (26). Current 2020 consensus guidelines from the American Society of Clinical Radiology (ASCO), American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO) recommend that patients with newly diagnosed breast cancer undergoing mastectomy and a mutation in a moderate-penetrance gene who are interested in CRRM should be offered the option of NSM as a reasonable oncologic option (7). In breast cancer patients with moderate-penetrance germline mutations such as PALB2, there is scarce evidence in the literature to help inform the clinical question of the role of contralateral risk-reducing NSM (7).
The recommendation for physicians to offer risk-reducing NSM to patients with PALB2 mutations is based on expert opinion and should take into account individualized breast cancer risk estimates tailored to the patient. The CanRisk Tool is a web interface for the latest version of the BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm), a comprehensive risk model used to calculate future risks of developing breast or ovarian cancer using family history, rare pathogenic variants in cancer susceptibility genes, PRSs, lifestyle and hormonal risk factors, and mammographic density (27). The BOADICEA model also computes the probabilities of carrying a pathogenic variant in BRCA1, BRCA2, PALB2, CHEK2 and ATM. The CanRisk Tool and similar probability models have been incorporated into the NCCN guidelines for familial breast/ovarian cancer and play an important role along with breast cancer stage and survival in decisions regarding risk-reducing surgery.
Recent literature suggests that PALB2 pathogenic variants increase the risk of developing ovarian cancer up to 5% and pancreatic cancer up to 2–3% by age 80 years (8). Prophylactic bilateral salpingo-oophorectomy is recommended in BRCA mutation carriers starting at the age of 35 and can reduce ovarian cancer risk by over 80% (11). However, not enough data exists to make this recommendation to PALB2 mutation carriers routinely. Updated NCCN guidelines have been revised from insufficient evidence for risk-reducing salpingo-oophorectomy (RRSO) and management based on family history to consideration of RRSO above age 45 years (10). Given the significant increase in ovarian cancer risk in women with PALB2 mutations after age 45 years, providers should consider recommending RRSO to postmenopausal PALB2 mutation carriers (7). Additionally, the latest NCCN guidelines recommend screening of PALB2 mutation carriers with a family history of pancreatic cancer with magnetic resonance cholangiopancreatography (MRCP)/contrast-enhanced MRI and/or endoscopic ultrasound (11).
Strengths of this study include the evaluation of a subpopulation that has not been widely studied, breast cancer patients who harbor a PALB2 pathologic variant, and analysis of the decision-making factors involved in choosing breast cancer risk-reducing surgery. The study is limited by its sample size of 5 patients and observational study design. Given the nature of the study, we are unable to make conclusions regarding the overall impact on oncologic outcomes and/or risk of complications from risk-reducing surgery without a larger number of patients and longer follow-up times. Further investigations with large retrospective and prospective clinical studies are necessary to determine the benefits, risks and outcomes of CRRM and/or risk-reducing bilateral salpingo-oophorectomy in this patient population.
Conclusions
The question of whether or not to perform a CRRM in a PALB2 patient with newly diagnosed breast cancer will remain unanswered until retrospective or prospective studies can be performed. Based on our limited experience and the currently available literature, we cannot make the recommendation for routine CRRM. However, we can report that both risk-reducing surgery and observation are viable options that have appeared to be safe in the majority of our patients. Given the inherent risks of undergoing risk-reducing mastectomy, we advocate for shared decision-making including a frank discussion of possible complications. In the setting of management guidelines that remain under development, the decision not only for risk-reducing mastectomy but also RRSO should be made on a case-by-case basis guided by individualized risk estimates based on age at diagnosis, family history and other risk factors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-33/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-33/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The case series conformed to the provisions of the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this study and accompanying images. Copies of the written consents are available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Couch FJ, Shimelis H, Hu C, et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol 2017;3:1190-6. [Crossref] [PubMed]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007;25:1329-33. [Crossref] [PubMed]
- Southey MC, Goldgar DE, Winqvist R, et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet 2016;53:800-11. [Crossref] [PubMed]
- Tischkowitz M, Balmaña J, Foulkes WD, et al. Management of individuals with germline variants in PALB2: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021;23:1416-23. [Crossref] [PubMed]
- Breast Cancer Association Consortium. Breast Cancer Risk Genes - Association Analysis in More than 113,000 Women. N Engl J Med 2021;384:428-39. [Crossref] [PubMed]
- Hu C, Hart SN, Gnanaolivu R, et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N Engl J Med 2021;384:440-51. [Crossref] [PubMed]
- Tung NM, Boughey JC, Pierce LJ, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol 2020;38:2080-106. [Crossref] [PubMed]
- Yang X, Leslie G, Doroszuk A, et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J Clin Oncol 2020;38:674-85. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER website, April 2020.
- Rosenthal ET, Evans B, Kidd J, et al. Increased Identification of Candidates for High-Risk Breast Cancer Screening Through Expanded Genetic Testing. J Am Coll Radiol 2017;14:561-8. [Crossref] [PubMed]
- Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf (Accessed on September 15, 2022).
- Song CV, Teo SH, Taib NA, et al. Surgery for BRCA, TP53 and PALB2: a literature review. Ecancermedicalscience 2018;12:863. [Crossref] [PubMed]
- Bergstrom C, Pence C, Berg J, et al. Clinicopathological Features and Outcomes in Individuals with Breast Cancer and ATM, CHEK2, or PALB2 Mutations. Ann Surg Oncol 2021;28:3383-93. [Crossref] [PubMed]
- Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med 2014;371:497-506. [Crossref] [PubMed]
- Heikkinen T, Kärkkäinen H, Aaltonen K, et al. The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggressive tumor phenotype. Clin Cancer Res 2009;15:3214-22. [Crossref] [PubMed]
- Ludwig KK, Neuner J, Butler A, et al. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg 2016;212:660-9. [Crossref] [PubMed]
- Metcalfe K, Eisen A, Senter L, et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br J Cancer 2019;121:15-21. [Crossref] [PubMed]
- Barton MB, West CN, Liu IL, et al. Complications following bilateral prophylactic mastectomy. J Natl Cancer Inst Monogr 2005;61-6. [Crossref] [PubMed]
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017;317:2402-16. [Crossref] [PubMed]
- Cybulski C, Kluźniak W, Huzarski T, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol 2015;16:638-44. [Crossref] [PubMed]
- Franceschini G, Di Leone A, Terribile D, et al. Bilateral prophylactic mastectomy in BRCA mutation carriers: what surgeons need to know. Ann Ital Chir 2019;90:1-2. [Crossref] [PubMed]
- Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat 2014;144:443-55. [Crossref] [PubMed]
- Lowry KP, Geuzinge HA, Stout NK, et al. Breast cancer screening for carriers of ATM, CHEK2, and PALB2 pathogenic variants: A comparative modeling analysis. J Clin Oncol 2021;39:10500. [Crossref]
- Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg 2017;225:361-5. [Crossref] [PubMed]
- Margenthaler JA, Gan C, Yan Y, et al. Oncologic Safety and Outcomes in Patients Undergoing Nipple-Sparing Mastectomy. J Am Coll Surg 2020;230:535-41. [Crossref] [PubMed]
- Jakub JW, Peled AW, Gray RJ, et al. Oncologic Safety of Prophylactic Nipple-Sparing Mastectomy in a Population With BRCA Mutations: A Multi-institutional Study. JAMA Surg 2018;153:123-9. [Crossref] [PubMed]
- Carver T, Hartley S, Lee A, et al. CanRisk Tool-A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol Biomarkers Prev 2021;30:469-73. [Crossref] [PubMed]
Cite this article as: Masanam MK, Pitcher CW, Sogunro O, Starks LK, Murray AB, Boisvert ME. Risk-reducing surgery in PALB2 mutations carriers with breast cancer: a case series and literature review. Transl Breast Cancer Res 2022;3:37.


