
A review of endocrine therapy in early estrogen receptor-positive breast cancer by St. Gallen Breast Cancer Conference 2021
Introduction
Estrogen receptor (ER) positive (+), human epidermal growth factor receptor 2 negative (HER2−) breast cancer is the most common type of breast cancer (1), and accounts for 65% of breast cancer in women aged under 50, and 75% of breast cancer in women aged 50 and over. ER+ breast cancer is heterogeneous; differences in ER expression level, progesterone receptor (PR) expression, histological grade, the degree of proliferation (Ki-67), and the type and frequency of genomic changes in different patients lead to different degrees of disease progression and prognoses. Thus, the corresponding therapeutic regimens for ER+ breast cancer should differ according to the individual characteristics of patients. At the 17th international St. Gallen Breast Cancer Conference (SG-BCC) in 2021, experts voted on numerous critical issues in relation to the use of neoadjuvant endocrine therapy (NET) and adjuvant endocrine therapy (AET) in the treatment of ER+ breast cancer. All clinicians and researchers should give consideration to the outcome of this poll.
In this article, we interpret the critical issues related to NET and AET according to the voting results of the 2021 SG-BCC and clinical practice in China. Specifically, we consider the following 8 points: (I) the selection of prognostic indicators for NET; (II) the selection of a NET population based on Ki-67; (III) the selection of polygenic testing combined with Ki-67 in the exemption of neoadjuvant chemotherapy; (IV) the selection of an AET population based on ER expression level and tumor size; (V) the selection of ovarian function suppression (OFS) for a premenopausal population; (VI) the selection of early stage intensive AET for a postmenopausal population; (VII) the selection of an extended AET population; and (VIII) the selection of individuals exempt from chemotherapy. The relevant questions and corresponding voting results for each point are set out in Table 1.
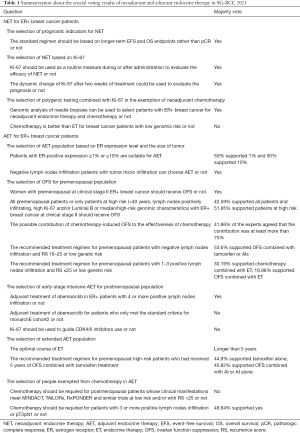
Full table
NET for ER+ breast cancer patients
The selection of prognostic indicators for NET
Pathologic complete response (pCR) has been used for more than a decade as an alternative endpoint for clinical trials and drug approval in early breast cancer; however, a majority of experts (83.05%) surveyed in the 2021 SG-BCC poll were of the view that the endpoints of the standard regimen should be longer-term event-free survival (EFS) and overall survival (OS) rather than pCR. In relation to luminal A breast cancer, a clinical study (2) showed that there was little difference between the prognoses of pCR and non-PCR patients, and thus pCR should not be used as a prognostic indicator. The development of new predictors associated with clinical outcomes is urgent and will provide opportunities to develop new strategies to reverse resistance to NET and identify response/resistance biomarkers in translational research.
The role of Ki-67 as a marker for evaluating the efficacy of NET
Ki-67 is a marker commonly used clinically to evaluate tumor proliferation status in breast cancer patients, and is easy to stain and score. However, currently, there is no consensus as to the threshold of Ki-67 in NET for breast cancer. ACOSCG Z1031, POETIC, and ADAPT state that the threshold of Ki-67 is 10%, while P024, PALLET and NeoMonarch state that the threshold of Ki-67 is 2.7%. Nearly 70% of experts surveyed in the 2021 SG-BCC poll agreed that Ki-67 should be used as a routine measure during or after the administration of NET to evaluate its efficacy, and that the dynamic change of Ki-67 after 2 weeks of treatment could be used to evaluate prognosis. Notably, the POETIC study (3) showed that the 5-year recurrence risk was 19.6% in those with a high baseline Ki-67 and a high Ki-67 after 2 weeks of ET, but only 8.9% in those with a high baseline Ki-67 and a low Ki-67 after 2 weeks of ET. The identification of high-risk populations is critical to subsequent treatment choices. In addition to Ki-67, the predictive effects of the Preoperative Endocrine Prognosis Index (PEPI) on the efficacy and recurrence risk of NET for ER+ breast cancer was verified by the FELINE and ALTERNATE studies.
The value of polygenic testing combined with Ki-67 in the exemption of neoadjuvant chemotherapy in ER+ breast cancer
The applications of Ki-67 monitoring in NET are not limited to those mentioned above. The ADAPT study (4) combined Ki-67 with genetic testing and showed that the 5-year invasive disease-free survival (iDFS) rate of patients at medium risk [with a recurrence score (RS) of 12–25] and a Ki-67 <10% within 3–4 weeks, who received ET without chemotherapy, reached 92.6%. Notably, this rate did not differ significantly from that of patients with a RS of 0–11, who had a 5-year iDFS of 93.9%. These results further highlight the significance of detecting dynamic changes of Ki-67 after NET. The ADAPT study also showed the ability of polygenic testing tools to assist in the screening of patients for neoadjuvant studies. Thus, more than 70% of experts surveyed at the 2021 SG-BCC were of the view that the genomic analysis of needle biopsies can be used to select patients with ER+ breast cancer for NET and chemotherapy, and more than 90% of experts were of the view that chemotherapy is no better than ET in the treatment of breast cancer patients with a low genomic risk.
AET for ER+ breast cancer patients
Threshold selection for AET population
In selecting patients to undergo AET, the threshold of ER+ expression is the key screening marker. 50% of experts surveyed in the2021 SG-BCC poll were of the view that patients with an ER+ expression ≥1% were suitable for AET, while the other 50% of experts were of the view that patients with an ER+ expression ≥10% were suitable for AET. The question of how to determine the ER+ expression threshold has been the focus of extensive AET research. In 2011, the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) guidelines (5) suggested that the threshold of ER+ expression should be 1%. A study (6) showed that patients who received chemotherapy with low ER expression (ER expression <10%) of HER2- breast cancer had a poor clinical prognosis, and that the time to recurrence (TTR) and OS rate of the group with an ER expression ≥10% were higher than those of the 1% ≤ ER <10% and ER <1% expression group (it should be noted there was no difference between these 2 groups). In terms of AET, a meta-analysis (7) showed no difference between the OS of patients with a low ER expression (1–9%) after 5 years of AET and the OS of patients who did not undergo ET (P=0.684). In the diagnosis and treatment process, Chinese doctors are of the view that patients with a 10% positive ER expression rate would benefit from ET, and 1% as the expansion of ET population makes patients not to miss the opportunity of ET.
In terms of the threshold selection of tumor size, more than 50% of experts surveyed in the 2021 SG-BCC poll were of the view that ER+ negative lymph node infiltration patients with tumor micro infiltration could choose AET either luminal A or luminal B. In contrast to the controversial issue of the selection of an ER+ threshold, most experts were of the view that an enlarged population is acceptable at the tumor size level of AET.
The proper application of intensive AET
OFS in the premenopausal population
In our clinical work, we have observed that Chinese breast cancer patients who attend our clinic are young in age. In relation to these premenopausal ER+ breast cancer patients, the choice of OFS is a topic of concern for many researchers. In the 2021 SG-BCC poll, more than 70% of experts agreed that premenopausal women at clinical stage II ER+ breast cancer should receive OFS, and more than 90% agreed that patients younger than 40 years old should receive OFS. However, experts’ opinions differed as to whether the entire premenopausal population or high-risk patients should receive OFS.
The SOFT (8) study provides insights into this issue. The SOFT study explored the efficacy of OFS and the efficacy of OFS combined with exemestane in premenopausal patients, and found no improvement in the 5-year disease-free survival (DFS) rates between the OFS combined with tamoxifen group and the tamoxifen monotherapy group; however, the benefits of breast cancer-free interphase and distant recurrence-free interphase (DRFI) were observed in the chemotherapy patients. Thus, OFS and chemotherapy appear to benefit patients at a high risk of recurrence. Following the results of the 8-year SOFT study (9), investigators observed a population-wide benefit in DFS when OFS was combined with tamoxifen, and an OS benefit in chemotherapy patients; however, no significant DRFI benefit was observed in patients who did not receive chemotherapy. In the 2021 SG-BCC poll, experts considered the possible contribution of chemotherapy-induced OFS to the effectiveness of chemotherapy overall, and more than 40% agreed that the contribution was more than 75%. The ASTRRA (10) study also confirmed the benefits of OFS for chemotherapy patients.
In the 2021 SG-BCC poll, experts’ opinions differed in relation to premenopausal patients with 1–3 positive lymph nodes and a RS of <25 or a low genetic risk. The combined analysis of the SOFT and TEXT studies (11) provides guidance on the selection of treatment combinations for premenopausal patients (OFS combined with exemestane or tamoxifen). Therefore, the questions of whether all premenopausal patients should receive OFS combined with aromatase inhibitors (AIs) and whether low-risk patients who do not undergo chemotherapy should choose OFS need to be considered when choosing therapeutic regimens for young patients with ER+ breast cancer.
CDK4/6 inhibitors in the postmenopausal population
AI is currently the gold standard of adjuvant therapy for postmenopausal ER+ early-stage breast cancer. Promising clinical trial results of CDK4/6 inhibitors in recent years have led to its rapid development in clinical use. Ongoing clinical studies include PENELOPE-B, PALLAS, monarchE and NATALEE (12). Among these, the monarchE study compared the efficacy of abemaciclib combined with standard ET, and a standard ET regimen, and found a 2-year iDFS of 92.3% (with a 3% absolute benefit) in the abemaciclib group. In the 2021 SG-BCC poll, more than 50% of experts supported the adjuvant treatment of abemaciclib in ER+ patients with 4 or more positive lymph nodes.
However, for patients who only met the standard criteria for monarchE cohort2, more than half of the experts did not support adjuvant therapy with abemaciclib. This relatively conservative opinion was similar to experts’ opinions on the use of the Ki-67 (in combination with other prognostic markers) to guide adjuvant therapy with CDK4/6 inhibitors. Notably, more than 60% of experts agreed that Ki-67 should not be used to guide CDK4/6 inhibitor use. In the monarchE subgroup, the high Ki-67 subgroup had a worse prognosis, and the abemaciclib-treated group had a higher 2-year iDFS than the ET alone group (91.3% vs. 86.1%).
There are several reasons why despite the encouraging results of the monarchE study experts failed to vote in favor of expanding the population for the early use of CDK4/6 inhibitors. First, in the studies of AET and CDK4/6 inhibitors mentioned above, a high risk was not the only factor affecting prognosis and remission. Second, the intensity of ET is not sufficient (monarchE used OFS in 21.7% of cases, tamoxifen in 30% of cases, and AI in 70% of cases). Third, the effect of current studies on advanced recurrence is not clear. The time to start CDK4/6 inhibitors after the recurrence of ER+ breast cancer 5 years later (about 50%) and the duration of the treatment are uncertain. Fourth, different CDK4/6 inhibitors may have different therapeutic effects on the intension of early AET due to different mechanisms. Researchers should continue to focus on the longer survival results of patients in these clinical studies to ensure the proper use of CDK4/6 inhibitors in the postmenopausal population.
Extending the duration of AET
In the 2021 SG-BCC poll, most experts supported a long course of ET that was not limited to 5 years. In relation to premenopausal high-risk patients who had received 5 years of OFS combined with tamoxifen treatment, most experts chose tamoxifen alone; however, some experts chose OFS combined with AI or AI alone. In relation to breast cancer patients with ER+ HER2- and positive lymph node infiltration, more than 80% of experts were of the view that the optimal course of ET was longer than 5 years. In relation to the use of prolonged ET, many clinical studies (13) have provided reference data; for example, ATLAS and ATTOM examined the efficacy of sequential tamoxifen after 5 years of tamoxifen treatment, NSABP B-33 studied sequential AI after 5 years of tamoxifen treatment, and NSABP B-42 studied sequential AI after 5 years of AI/tamoxifen treatment. These studies all found that, to varying degrees, the intensification of AET can lead to a breakthrough in extending the duration of treatment.
The selection of chemotherapy exempted from AET
In the 2021 SG-BCC poll, the issue of whether patients with a low risk of recurrence should be exempted from chemotherapy was also a key point. Overall patients in 3 related studies (MINDACT, TAILORx and RxPONDER) (14) were found not to benefit from chemotherapy, and nearly 80% of experts did not support chemotherapy in postmenopausal patients whose clinical manifestations met those of MINDACT, TAILORx, RxPONDER and similar trials at low risk and/or with a RS <25. However, experts’ opinions changed when the patient population was segmented. In relation to patients with 3 or more positive lymph nodes or pT3pN1, more than 40% of the experts were of the view that this patient population required chemotherapy. Thus, even in low-risk patients with a RS of <25, the decision as to whether chemotherapy is not necessary in the treatment regimen must be decided on a case-by-case basis.
Conclusions
In this review, we summarized and discussed the crucial issues considered at the 2021 SG-BCC, including how to administer chemotherapy to patients with different recurrence risks and how to properly use CDK4/6 inhibitors in postmenopausal patients and OFS in premenopausal patients undergoing adjuvant therapy for ER+ breast cancer. In relation neoadjuvant therapy, clinicians need to pay attention to the questions of how to predict the prognosis of patients according to the dynamic changes of Ki-67 and the duration of intensive ET. A series of clinical studies in the field of NET and AET have provided evidence for the selection of therapeutic screening criteria and effective prognostic markers by subdividing the efficacy evaluation results into population groups. Overall, in the process of ET for ER+ breast cancer, we should always balance individualized therapy and standardized therapy, and pay attention to progress in relevant clinical research.
Acknowledgments
We would like to thank L. Huleatt as an English language editor for the linguistic refinement of our manuscript.
Funding: The authors receive the fund from National Natural Science Foundation of China (No. 82002794).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tbcr-21-11). JY serves as the unpaid editorial board member of Translational Breast Cancer Research from May 2021 to April 2023. The authors report that they receive the fund from National Natural Science Foundation of China (No. 82002794). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055 [Crossref] [PubMed]
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-804. [Crossref] [PubMed]
- Smith I, Robertson J, Kilburn L, et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol 2020;21:1443-54. [Crossref] [PubMed]
- Robertson JFR, Evans A, Henschen S, et al. A Randomized, Open-label, Presurgical, Window-of-Opportunity Study Comparing the Pharmacodynamic Effects of the Novel Oral SERD AZD9496 with Fulvestrant in Patients with Newly Diagnosed ER+ HER2- Primary Breast Cancer. Clin Cancer Res 2020;26:4242-9. [PubMed]
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-95. [Crossref] [PubMed]
- Fujii T, Kogawa T, Dong W, et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol 2017;28:2420-8. [Crossref] [PubMed]
- Chen T, Zhang N, Moran MS, et al. Borderline ER-Positive Primary Breast Cancer Gains No Significant Survival Benefit From Endocrine Therapy: A Systematic Review and Meta-Analysis. Clin Breast Cancer 2018;18:1-8. [Crossref] [PubMed]
- Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015;372:436-46. [Crossref] [PubMed]
- Francis PA, Pagani O, Fleming GF, et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med 2018;379:122-37. [Crossref] [PubMed]
- Kim HA, Ahn SH, Nam SJ, et al. The role of the addition of ovarian suppression to tamoxifen in young women with hormone-sensitive breast cancer who remain premenopausal or regain menstruation after chemotherapy (ASTRRA): study protocol for a randomized controlled trial and progress. BMC Cancer 2016;16:319. [Crossref] [PubMed]
- Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014;371:107-18. [Crossref] [PubMed]
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020;38:3987-98. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol 2019;37:423-38. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med 2019;380:2395-405. [Crossref] [PubMed]
Cite this article as: Zhou Y, Yang J. A review of endocrine therapy in early estrogen receptor-positive breast cancer by St. Gallen Breast Cancer Conference 2021. Transl Breast Cancer Res 2021;2:20.


