
Presentation of metastatic breast cancer as a large bowel obstruction in an elderly female with resultant diverting ileostomy: case report
Introduction
Breast cancer is one of the leading causes of cancer in women. One in eight women will develop breast cancer in her lifetime and it is the leading cause of cancer-related death in women worldwide (1,2). Patients with breast cancer infrequently develop distant disease in other organs (only 5–15% of patients). Distant metastases occur most frequently in bone, lungs, or liver (3). In only 3.4–4.5% of patients will metastatic disease be identified in the colon. In these cases, this distant disease is frequently mistaken for a primary colorectal tumor (1,4). To our knowledge, there are no reports in the literature of the surgical management of elderly patients presenting with metastatic breast cancer as a large bowel obstruction. In this report, we present the unique case of an elderly female with a remote history of adequately treated breast cancer in her hometown of Lebanon, who presents with a large bowel obstruction secondary to breast cancer metastasis, ultimately treated with diverting ileostomy for palliation of her obstructive symptoms. We present the following case in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-27/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
An 84-year-old otherwise healthy female presented for screening mammogram in her hometown of Lebanon in November of 2016 and was found to have an area of calcifications, concerning for malignancy, in the right breast. Subsequent targeted right breast and axillary ultrasound revealed a large, greater than 5 cm, breast mass and an abnormal axillary lymph node. Ultrasound-guided biopsy of both the right breast mass and the abnormal axillary lymph node revealed invasive lobular carcinoma, estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor 2 receptor (HER2)/Ki. For this clinical T3N3aM0, she underwent bilateral mastectomy and right axillary lymph node dissection in January of 2017. Twenty-nine of 32 lymph nodes were positive for metastatic lobular carcinoma. There were negative margins on the mastectomy specimen. The patient then received adjuvant chemotherapy for 8 cycles with Adriamycin and Cyclophosphamide, followed by a taxane April to August of 2017. She tolerated this well without significant side effects or complications. Following adjuvant chemotherapy, the patient received 30 fractions of local radiation treatments to the right chest wall and axilla in late 2017, although the duration of radiation therapy is unknown. She completed 5 years of adjuvant endocrine therapy with letrozole, ending in early 2022.
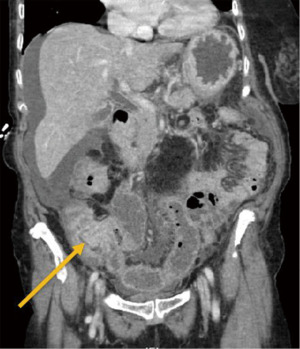
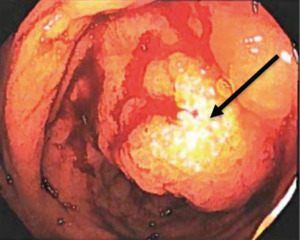
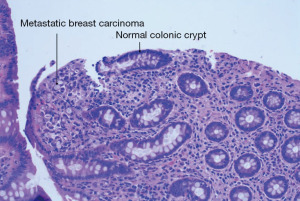
Five and a half years after her initial breast cancer diagnosis in March of 2022, the patient is living in the United States and presented to the hospital complaining of dark stools, abdominal pain, nausea, and vomiting for 2 weeks. On physical examination, her abdomen was mildly distended with voluntary guarding, and tender to palpation along the entire right abdomen with hypoactive bowel sounds. A computed tomography (CT) scan of her abdomen and pelvis (Figure 1) revealed ileocecal valve thickening, ascending colonic wall thickening with decompressed transverse and left colon, and scattered prominent lymph nodes within the mesentery, suggestive of a proximal large bowel obstruction. At this time, the patient was hemodynamically stable and not acutely obstructed and she therefore next underwent a diagnostic colonoscopy which revealed a polypoid partially obstructing circumferential mass in the ascending colon (Figure 2). Biopsies were taken and final pathology revealed metastatic breast cancer, lobular (signet ring) morphology (Figure 3). Given that she remained hemodynamically stable without clinical evidence of obstruction, she was discharged home and instructed to follow up with medical oncology, and to also follow up with colorectal surgery if obstructive symptoms returned.
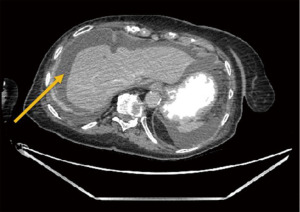
One week later in March of 2022, the patient presented to the colorectal surgery clinic and her abdomen was found to be severely distended, diffusely tender to palpation, with voluntary guarding on physical exam. She was sent for CT imaging and found to have evidence of a distal small bowel versus a proximal large bowel obstruction with possible internal hernia (Figures 4,5). Given her worsening physical exam and concerning CT findings, the patient was emergently taken to the operating room for exploratory laparotomy. Upon entry into the abdomen, the small bowel was diffusely dilated and there was evidence of omental studding and carcinomatosis. Given the evidence of widespread intra-abdominal carcinomatosis and metastatic breast cancer, the patient underwent a diverting loop ileostomy to manage her proximal large bowel obstruction. She recovered well from the procedure and was ultimately discharged home to family with plans to follow up with medical oncology for palliative chemotherapy and anti-hormone therapy treatment to begin in June of 2022. The patient has since followed in the colorectal surgery clinic with a well-functioning ileostomy and without further abdominal symptoms. Her ongoing management will consist of chemotherapy and anti-hormone therapy, and without any further plans for surgical intervention. A complete timeline of the events in the patient’s breast cancer history is illustrated in Figure 6.

Discussion
Despite treatment, more than half of breast cancer patients experience some form of tumor recurrence or metastasis during their lifetime (1). When breast cancer metastasizes, it most commonly will spread to the lymph nodes, lung, liver, or bone (1,3). Metastasis to the gastrointestinal (GI) tract is much less common, affecting only 3.4–4.5% of patients, with the majority of these cases being associated with invasive lobular carcinoma (4.5% of cases in comparison to 0.2% of cases of invasive ductal carcinoma) (1,3,5). The proposed mechanism for this is related to the creation of a hospitable environment by inflammatory cells, chemokines, and pathogens (6). When involvement of the GI tract does occur, the stomach is primarily affected, closely followed by the small bowel (5,7). Available data indicate that colonic metastasis from invasive breast cancer makes up only 3% of cases (7). Patients, in this setting, may present with a variety of symptoms including abdominal pain, nausea, vomiting, early satiety, weight loss, or GI bleeding, and imaging findings may show evidence of stenosis, mucosal irregularity, or diffuse colonic wall thickening (1,3,5). These symptoms and findings present 6–9 years, on average, after initial breast cancer diagnosis and treatment, though some cases have occurred at an interval of up to 30 years from initial diagnosis and presentation (3,5).
When GI metastasis from invasive breast cancer is identified, surgical management is generally reserved for palliative purposes only, usually in the setting of acute obstruction, bleeding, or perforation, and is not associated with an overall survival benefit (7,8). With other metastatic malignancies such as ovarian cancer, surgical debulking prior to institution of systemic therapy is recommended (6). Instead, the goals of treatment of metastatic breast cancer focus on providing systemic therapies to prolong survival, alleviate symptoms, and maintain quality of life. For patients with hormone receptor positive, HER2 negative disease, first line therapy is generally initiated with endocrine therapy. Systemic cytotoxic chemotherapy is generally reserved for visceral metastases with end-organ dysfunction or endocrine-refractory disease. Unfortunately, the average survival after diagnosis is only 12 months (9,10).
In this report, we describe the case of an elderly patient with remote history of invasive lobular carcinoma, treated with bilateral mastectomy, axillary dissection, adjuvant chemotherapy, radiation, and anti-hormone therapy, who presented five and a half years later with metastatic breast cancer in the right colon, presenting as large bowel obstruction. Though exceedingly uncommon, breast cancer can metastasize to the GI tract, including the colon, up to 30 years from initial treatment. Generally, this is related to an underlying inflammatory or infectious process creating an inviting environment for tumor cells to thrive (10). Given our patient’s presentation and what is known in the literature about metastatic spread of breast cancer to the gastrointestinal tract, it is likely that this patient developed some inciting inflammatory or infectious event in the colon, since her initial diagnosis, which was inviting to tumor cells and precipitated metastatic spread. In the setting of appropriately treated breast cancer with anti-hormone therapy, surgery, and adjuvant chemotherapy and radiation, this is certainly interesting, and illustrates that even appropriately treated breast cancer may still have metastatic potential. This patient also illustrates the importance of considering metastatic disease in patients with a history of prior malignancy who present with obstructive symptoms. Failing to do so can result in a delay in recognition of metastatic tumor biology or even a misdiagnosis, which can lead to delays in appropriate treatment and significant morbidity or even mortality for patients. It is known that patients with metastatic breast cancer tumor cells typically have a shorter life span (11-13), but it is possible to live a long life after the diagnosis of metastatic breast cancer. According to some studies, once distant metastasis is recognized, patients generally live only another 2–3 years (14); while other studies show that up to 25% of metastatic breast cancer patients are alive 5 years after metastasis is found and treated (15).
There are several limitations to this study. Given that this is a single patient case report, there are limits to the generalizability of this report as a result of the low sample size. This study is also retrospective in nature and it is therefore difficult to deduce causal relationships. Additionally, this patient had her primary surgery in Lebanon and obtaining her records was limited and much of this history was obtained through patient report, which may have some underlying inherent bias. However, this case study is of value to the literature by including an elderly patient, a patient population that is largely absent in the literature, and demonstrates surgical treatment options for palliation of symptoms in metastatic breast cancer presenting as a large bowel obstruction. Given that this patient recovered well, other patients with similar presentation may benefit from similar management.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-27/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noor A, Lopetegui-Lia N, Desai A, et al. Breast Cancer Metastasis Masquerading as Primary Colon and Gastric Cancer: A Case Report. Am J Case Rep 2020;21:e917376. [Crossref] [PubMed]
- Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199-209. [Crossref] [PubMed]
- Khan I, Malik R, Khan A, et al. Breast Cancer Metastases to the Gastrointestinal Tract Presenting with Anemia and Intra-abdominal Bleed. Cureus 2017;9:e1429. [Crossref] [PubMed]
- Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol 1998;93:111-4. [Crossref] [PubMed]
- Nazareno J, Taves D, Preiksaitis HG. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol 2006;12:6219-24. [Crossref] [PubMed]
- Gnerlich J, Jeffe DB, Deshpande AD, et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Ann Surg Oncol 2007;14:2187-94. [Crossref] [PubMed]
- Uygun K, Kocak Z, Altaner S, et al. Colonic metastasis from carcinoma of the breast that mimics a primary intestinal cancer. Yonsei Med J 2006;47:578-82. [Crossref] [PubMed]
- Samo S, Sherid M, Husein H, et al. Metastatic infiltrating ductal carcinoma of the breast to the colon: a case report and literature review. Case Rep Gastrointest Med 2013;2013:603683. [Crossref] [PubMed]
- Zhou XC, Zhou H, Ye YH, et al. Invasive ductal breast cancer metastatic to the sigmoid colon. World J Surg Oncol 2012;10:256. [Crossref] [PubMed]
- Villa Guzmán JC, Espinosa J, Cervera R, et al. Gastric and colon metastasis from breast cancer: case report, review of the literature, and possible underlying mechanisms. Breast Cancer (Dove Med Press) 2017;9:1-7. [Crossref] [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer 2015;112:1445-51. [Crossref] [PubMed]
- Park M, Kim D, Ko S, et al. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int J Mol Sci 2022;23:6806. [Crossref] [PubMed]
- Sundquist M, Brudin L, Tejler G. Improved survival in metastatic breast cancer 1985-2016. Breast 2017;31:46-50. [Crossref] [PubMed]
- Samiee S, Berardi P, Bouganim N, et al. Does removal of the primary tumor in patients with metastatic breast cancer improve either local control or overall survival? J Clin Oncol 2011;29:e11511. [Crossref]
Cite this article as: Greenseid S, Staudinger K, Morgan R, Blake K. Presentation of metastatic breast cancer as a large bowel obstruction in an elderly female with resultant diverting ileostomy: case report. Transl Breast Cancer Res 2022;3:38.



