
Pyrotinib: a new promising targeted agent for human epidermal growth factor receptor 2-positive breast cancer
Approximately 20% of all new breast cancer cases are characterized by the positivity for the human epidermal growth factor receptor 2 (HER2) in terms of gene amplification or overexpression of its protein (1). Despite being characterized by an aggressive behavior and poorer prognosis, the advent of anti-HER2 targeted therapies has dramatically improved the survival of patients affected by this disease subtype over the last two decades (2). Nowadays, in the advanced setting, patients with HER2-positive breast cancer are those who are administered with more lines of treatment and with the expected longer survival outcomes as compared to patients affected by other subtypes (3,4). To further improve the outcomes of patients with HER2-positive metastatic breast cancer and possibly increase the chances of cure even in the presence of advanced disease, developing new effective targeted treatment options remains a priority (5).
The current standard of care in the first-line setting of HER2-positive metastatic breast cancer is represented by chemotherapy with a taxane as single agent in combination with dual anti-HER2 blockade including the monoclonal antibodies pertuzumab plus trastuzumab (6,7). This regimen has shown an extraordinary and unprecedented overall survival (OS) gain in this setting (8). Following treatment with trastuzumab plus a taxane, the antibody drug-conjugate trastuzumab emtansine (T-DM1) represents the current recommended approach (9), although more limited evidence on its performance exists in patients previously exposed also to pertuzumab (10). Based on current guidelines, the tyrosine kinase inhibitor (TKI) lapatinib in combination with capecitabine represents one of the available third-line treatment options (6,7).
In recent years, several other TKIs have been developed for patients with HER2-positive metastatic breast cancer (Table 1) (11-22).
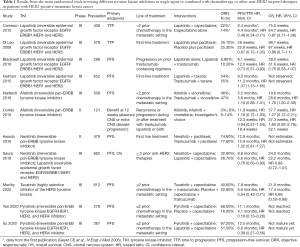
Full table
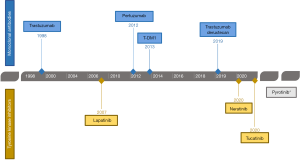
Among the different TKIs, in addition to lapatinib, also neratinib and tucatinib have been recently approved by the Food and Drug Administration (FDA) thus becoming two new available targeted treatment options for patients with HER2-positive metastatic breast cancer (Figure 1).
The NALA trial compared a combination of neratinib plus capecitabine versus lapatinib plus capecitabine in patients affected by HER2-positive metastatic breast cancer previously treated with at least two lines of therapy (11). The study showed a significant improvement in progression-free survival (PFS) favoring neratinib [mean PFS 8.8 vs. 6.6 months; hazard ratio (HR) 0.76, 95% confidence interval (CI): 0.63–0.93, P=0.0059] without difference in OS (mean 24.0 vs. 22.2 months; HR 0.88, 95% CI: 0.72–1.07, P=0.2086) (11).
Important results have more recently become available from the HER2CLIMB trial that investigated the addition of tucatinib to capecitabine and trastuzumab. A significant improvement in both PFS (median 7.8 vs. 5.6 months; HR 0.54, 95% CI: 0.42–0.71, P<0.001) and OS (21.9 vs. 17.4 months; HR 0.66, 95% CI: 0.50–0.88, P=0.005) was observed with the addition of the TKI (13). Moreover, this regimen proved to be highly effective also for patients with brain metastasis (23). Notably, in this trial all patients were previously treated with trastuzumab, pertuzumab, and T-DM1 (13).
In the current issue of Translational Breast Cancer Research, Yan and colleagues reports the results of the PHENIX phase III trial (24). In this study, the efficacy of a new TKI, pyrotinib (an irreversible pan-HER inhibitor targeting EGFR, HER2 and HER4), was investigated in patients with HER2-positive metastatic breast cancer previously treated with up to two prior lines of therapy (that included also trastuzumab plus a taxane). This is a double-blinded, placebo-controlled phase III study conducted in China that enrolled 279 patients who were randomized (2:1) to receive 21-day cycle of either oral pyrotinib or placebo (400 mg, qd) combined with capecitabine (1,000 mg/mq, bid on days 1–14). Patients progressing on placebo plus capecitabine could receive subsequent pyrotinib monotherapy. The primary endpoint was PFS; secondary endpoints included OS, disease control rate (DCR), clinical benefit rate (CBR), duration of response (DoR) and safety.
Approximately 54% of patients had hormone receptor-positive disease and 78% had visceral involvement. A total of 11% of patients in both arms had brain metastases. All patients received prior trastuzumab in the early and/or advanced setting, but only 63% were exposed to this monoclonal antibody for metastatic disease. Notably, in terms of prior lines of therapy received for advanced disease, 34% of patients were treated in the first-line setting.
In the pyrotinib and control groups, respectively, median PFS was 11.1 and 4.1 months (HR 0.18, 95% CI: 0.13–0.26, P<0.001). The objective response rate (ORR) rate was 68.6% and 16.0% in the pyrotinib and control groups, respectively (P<0.001). Notably, 12 patients achieved a complete response in the pyrotinib group, while none in the control group. Both DCR (from 64.9% to 91.9%, P<0.001) and CBR (from 22.3% to 76.8%, P<0.001) were also significantly improved by the addition of pyrotinib. OS could not be assessed due to the limited number of events observed (41 deaths were registered: 23 in the pyrotinib group and 18 in the control group).
As regards to patients with visceral disease, improvement in PFS were observed in both patients with or without brain metastases that received pyrotinib (6.9 vs. 4.2 months and 11.1 vs. 4.1 months, respectively). New brain metastases were developed in 1.2% of patients in the pyrotinib group and 3.6% of those in the control group.
A total of 71 patients received pyrotinib monotherapy after progressing on placebo plus capecitabine: promising activity was also observed in this setting with median PFS of 5.5 months, ORR of 38%, DCR of 80.3% and CBR of 42.3%.
In terms of safety profile, patients in the pyrotinib plus capecitabine arm experienced higher rates of treatment-related adverse events (TRAEs) of any grade than those on placebo plus capecitabine arm (99.5% vs. 95.7%). The most common observed side effects were diarrhea (98.4% vs. 68.1%), hand and foot syndrome (59.5% vs. 29.8%), nausea (48.6% vs. 18.1%) and vomiting (48.6% vs. 16.0%). A total of 55.1% of patients in the pyrotinib arm and 25.5% in the control arm developed Grade 3 and 4 TRAEs. Diarrhea was the most frequent Grade 3 TRAE (30.8% and 12.8%, respectively) followed by hand and foot syndrome (15.7% and 5.3%, respectively).
The PHENIX trial has provided important data on the potential use of a new TKI for the treatment of patients with HER2-positive metastatic breast cancer. Two important considerations should be made when placing the results of the trial in the current anti-HER2 treatment era. The comparator arm was chemotherapy alone without targeted anti-HER2 therapy and the patients included were not heavily pretreated being exposed only to prior trastuzumab plus taxane-based chemotherapy.
Regarding the first issue, a new trial addressing this concern has been recently presented at the 2020 American Society of Clinical Oncology (ASCO) Annual Conference. In the PHOEBE trial, patients affected by metastatic HER2-positive breast cancer previously exposed to no more than 2 lines of therapy including also trastuzumab and taxane-based chemotherapy were randomly assigned to receive either pyrotinib (400 mg/day) or lapatinib (1,250 mg/day), both given with capecitabine (1,000 mg/m2). The primary endpoint was PFS. Median PFS in the pyrotinib plus capecitabine arm was 12.5 vs. 6.8 months in the lapatinib plus capecitabine arm (HR 0.39, 95% CI: 0.27–0.56, P<0.001). OS data were not mature yet; however, a trend for improved OS favoring the pyrotinib arm was observed. In terms of safety profile, Grade 3 diarrhea was experienced by 30.6% of patients in the pyrotinib arm as compared to 8.3% in the lapatinib arm (22).
Regarding the second issue, both the PHENIX and PHOEBE trials included a patient population that is currently not candidate yet to an anti-HER2 TKI according to current guidelines. The performance of pyrotinib following pertuzumab-based therapy and T-DM1 remains unknown. In addition, with the availability of other important TKIs, such as tucatinib and neratinib, it would be important to investigate the efficacy of pyrotinib also in a more pretreated patient population to give a potential additional effective option in later lines.
Considering that HER2-positive metastatic breast cancer remains an incurable disease and resistance to the different available treatments occurs at some point, developing further effective agents for these patients remains a priority. In the last year, several steps forward have been made with the FDA approval of two TKIs and of the new antibody drug-conjugate trastuzumab-deruxtecan (Figure 1). Other promising strategies are currently being studied. Among them, promising results have been obtained with margetuximab, a new Fc-engineered anti-HER2 monoclonal antibody (25). In addition, several new combination strategies of anti-HER2 targeted agents are currently being developed, including with CDK4/6 inhibitors (26), phosphoinositide 3-kinase (PI3K) inhibitors (27) as well as immunotherapy (28).
In conclusion, the continuous successful advances in anti-HER2 targeted therapies has significantly improved the prognosis of patients with HER2-positive metastatic breast cancer. More treatment lines are now available for these patients. Pyrotinib represents another promising option that is already approved for clinical use in China. More data are needed to better understand where this strategy can be placed in the current treatment algorithm for patients with HER2-positive metastatic breast cancer.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tbcr-20-34). ML reports that he acted as consultant for Roche and Novartis, and received honoraria from Theramex, Takeda, Roche, Lilly, Pfizer and Novartis outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009;14:320-68. [Crossref] [PubMed]
- Lambertini M, Pondé NF, Solinas C, et al. Adjuvant trastuzumab: a 10-year overview of its benefit. Expert Rev Anticancer Ther 2017;17:61-74. [Crossref] [PubMed]
- Seah DSE, Luis IV, Macrae E, et al. Use and Duration of Chemotherapy in Patients with Metastatic Breast Cancer According to Tumor Subtype and Line of Therapy. J Natl Compr Canc Netw 2014;12:71-80. [Crossref] [PubMed]
- Deluche E, Antoine A, Bachelot T, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer 2020;129:60-70. [Crossref] [PubMed]
- Lambertini M, Vaz-Luis I. Is HER2-positive metastatic breast cancer still an incurable disease? Lancet Oncol 2020;21:471-2. [Crossref] [PubMed]
- Giordano SH, Temin S, Chandarlapaty S, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:2736-40. [Crossref] [PubMed]
- Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol 2018;29:1634-57. [Crossref] [PubMed]
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519-30. [Crossref] [PubMed]
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732-42. [Crossref] [PubMed]
- Conte B, Fabi A, Poggio F, et al. T-DM1 Efficacy in Patients With HER2-positive Metastatic Breast Cancer Progressing After a Taxane Plus Pertuzumab and Trastuzumab: An Italian Multicenter Observational Study. Clin Breast Cancer 2020;20:e181-7. [Crossref] [PubMed]
- Saura C, Oliveira M, Feng YH, et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J Clin Oncol 2019;37:abstr 1002.
- Awada A, Colomer R, Inoue K, et al. Neratinib Plus Paclitaxel vs Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer: The NEfERT-T Randomized Clinical Trial. JAMA Oncol 2016;2:1557. [Crossref] [PubMed]
- Murthy RK, Loi S, Okines A, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med 2020;382:597-609. [Crossref] [PubMed]
- Harbeck N, Huang CS, Hurvitz S, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol 2016;17:357-66. [Crossref] [PubMed]
- Cortés J, Dieras V, Ro J, et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol 2015;16:1700-10. [Crossref] [PubMed]
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N Engl J Med 2006;355:2733-43. [Crossref] [PubMed]
- Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol 2008;26:5544-52. Erratum in: J Clin Oncol. 2009 Apr 10;27(11):1923. [Crossref] [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010;28:1124-30. [Crossref] [PubMed]
- Cameron D, Casey M, Oliva C, et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 2010;15:924-34. [Crossref] [PubMed]
- Gelmon KA, Boyle FM, Kaufman B, et al. Lapatinib or Trastuzumab Plus Taxane Therapy for Human Epidermal Growth Factor Receptor 2-Positive Advanced Breast Cancer: Final Results of NCIC CTG MA.31. J Clin Oncol 2015;33:1574-83. [Crossref] [PubMed]
- Jiang Z, Yan M, Hu X, et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: A randomized phase III study. J Clin Oncol 2019;37:abstr 1001.
- Xu B, Yan M, Ma F, et al. Pyrotinib or lapatinib plus capecitabine for HER2+ metastatic breast cancer (PHOEBE): A randomized phase III trial. J Clin Oncol 2020;38:abstr 1003.
- Lin NU, Borges V, Anders C, et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol 2020. Epub ahead of print. [Crossref] [PubMed]
- Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebocontrolled phase 3 study. Transl Breast Cancer Res 2020;1:13. [Crossref]
- Rugo HS, Im SA, Cardoso F, et al. Abstract GS1-02: Phase 3 SOPHIA study of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: second interim overall survival analysis. In: General Session Abstracts. American Association for Cancer Research, 2020.
- Tolaney SM, Wardley AM, Zambelli S, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol 2020;21:763-75. [Crossref] [PubMed]
- Jain S, Shah AN, Santa-Maria CA, et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat 2018;171:371-81. [Crossref] [PubMed]
- Loi S, Giobbie-Hurder A, Gombos A, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol 2019;20:371-82. [Crossref] [PubMed]
Cite this article as: Perachino M, Arecco L, Martelli V, Lambertini M. Pyrotinib: a new promising targeted agent for human epidermal growth factor receptor 2-positive breast cancer. Transl Breast Cancer Res 2020;1:11.


