
Neoadjuvant chemotherapy versus surgery as the initial option for T3 breast cancer (>5 cm): real-world evidence from the Chinese Society of Clinical Oncology Breast Cancer database
Introduction
The initial purpose of neoadjuvant chemotherapy (NAC) in breast cancer was to downstage locally advanced cancer or to render inoperable cancer operable. Its application was then extended to early breast cancer to enable breast-conserving surgery. Now, it has become widely accepted for use in aggressive subtypes like stage II or III and human epidermal receptor growth factor 2 (HER2)-positive or triple-negative breast cancer, particularly for large tumor lesions (1).
NAC thus provides another therapy option for T3 patients, even though there is a lack of evidence definitively proving initial NAC to be superior to initial surgery. Initial NAC can presumably provide early eradication of large tumor lesions that would lighten the tumor load and decrease the risk of metastatic spread for T3 patients. It might also offer useful information concerning the drug sensitivity of different regimens, helping to guide subsequent therapy selection. Thus, there is urgent need to produce the clinical evidence that would confirm the benefit of initial NAC in breast cancer patients.
Recently, the American Food and Drug Administration (FDA) has considered data from real-world evidence (RWE) and from randomized clinical trial (RCTs) as complementary. The FDA is now working hard to collect data from electronic health records, billing data, and other sources (2). In the last past 2 years, we have been committed to establishing the Chinese Breast Cancer Database which was founded by the Chinese Society of Clinical Oncology of Breast Cancer (CSCO BC). Using this real-world data, we previously explored the difference in trastuzumab use in resource-limited versus resource-abundant regions and its survival benefit on HER2-positive breast cancer patients in China (CSCO BC RWS 15001) (3). For the present study, we used this database to evaluate the real-world treatment of T3 patients and determined whether NAC is better than surgery as an initial option for T3 breast cancer (CSCO BC RWS 17001). We present the following article in accordance with the STROBE reporting checklist. (available at http://dx.doi.org/10.21037/tbcr-20-21).
Methods
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was approved by the Institutional Review Board of the Affiliated Hospital of Qingdao University. Informed consent was obtained from all individual participants included in the study.
Patients and population
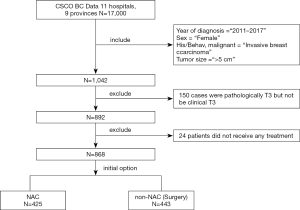
In December 2015, we established the first multicenter database funded by the CSCO BC. As of November 2017, information from more than 17,000 patient cases had been added to the CSCO BC database. These data were collected from more than 11 hospitals in 9 provinces across eastern China. For the current study, eligible cases needed to meet the following criteria: early stage invasive breast cancer (EBC) diagnosed from 2011 to 2017; maximum diameter of tumor larger than 5 centimeters (T3) irrespective to clinical examination; clearly clinical or pathological axillary node staging; NAC or breast cancer surgery received as the initial therapeutic strategy.
For inclusion, patients who received NAC as their initial therapy were required to receive at least two cycles of chemotherapy, while those who underwent breast-conserving surgery or mastectomy could not have also received NAC. Patients with borderline, unknown, or missing information for treatment were excluded (CSCO BC RWS 1701).
Outcome measures
The demographic and clinical characteristics of the two groups (the NAC group vs. non-NAC group) were summarized and compared. The primary endpoint of this study was event-free survival (EFS). We calculated EFS as the interval from randomization to the earliest occurrence of disease progression resulting in inoperability, locoregional recurrence (after NAC), distant metastasis, or death from any cause. Information from patients who were alive with no events occurring as of the analysis cutoff date were censored at the last follow-up date. The second endpoint was surgery method, with a focus on the breast-conserving rates of the two groups.
Statistical analysis
In this real world study, we compared the characteristic differences between the two groups using Pearson’s test. We also used Kaplan-Meier and Cox proportional hazards regression to estimate hazard ratio (HR) and 95% confidence interval (CI) for the relationship between different initial therapeutic strategies and EFS. P values <0.05 were considered statistically significant; all tests were two-sided. Statistical analysis was carried out using SPSS 20.0.
Results
Demographic characteristics
After applying the inclusion and exclusion criteria, data from 868 cases diagnosed as clinical T3 breast cancer were collected from the CSCO BC database (Figure 1). Table 1 shows clinical and demographic characteristics of these patients. Only 49.0% (425/868) of patients chose NAC after diagnosis (NAC group), and the remaining patients (51.0%) chose surgery as their initial therapeutic strategy (non-NAC group). Patients in the NAC group had more clinical positive lymph nodes (82.1% vs. 55.1%, P<0.001), more HER2-positive tumors (54.1% vs. 44.0%, P<0.05) and hormone receptor (HR)-negative tumors (23.8% vs. 16.3%, P<0.05) compared with the non-NAC group. However, Patients in the non-NAC group had more luminal subtype tumors (43.1% vs. 33.4%, P<0.05) compared with the NAC group (Table 1). There were no different with triple negative breast cancer in two groups (5.2% vs. 5.6%, P>0.05).
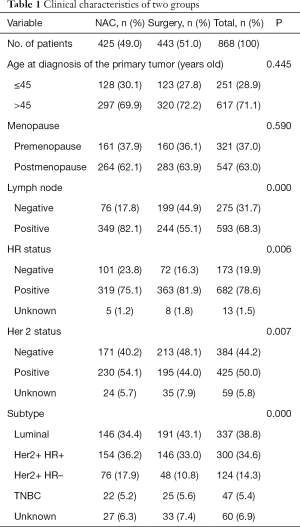
Full table
Treatment
There was no difference in surgery category between the two groups. Most patients of both groups chose mastectomy and axillary lymph node dissection (ALND), and fewer than 5% of patients chose breast-conserving surgery. In addition, 168 (39.5%) patients in the NAC group and 343 (77.4%) patients in the non-NAC group underwent adjuvant chemotherapy. Overall, 70 out of 425 patients (16.5%) achieved breast pathologic complete response (pCR) (Table 2).
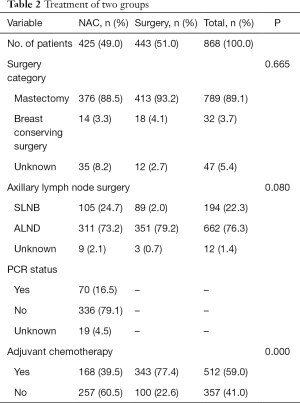
Full table
Overestimation and underestimation of tumor lesion
The accuracy rate between iconography and histopathology was examined, and, to avoid the influence from chemotherapy, only patients in the non-NAC group were included, as they all received surgery first. For these patients, 573 were diagnosed as T3 clinically or pathologically or by both methods. However, 443 of these patients (77.3%) were diagnosed as clinical T3, while 130 patients were diagnosed as pathological T3 but not be clinical T3. The underestimation of clinical examination was as high as 22.7% (85/573). Of these 443 patients, only 87.1% (386/443) were pathologic T3 after surgery, and the overestimation of clinical examination was 12.9% (57/443).
Survival estimates
The difference of survival between the two groups was analyzed, with a total of 257 events being recorded among these patients. The NAC group had a median time for EFS of 55 months, while the non-NAC group had a median time of 43 months, with no significance between the two groups (HR =0.82, 95% CI: 0.64–1.05) (Figure 2A). Furthermore, clinical lymph node-negative or HR-positive or HER2-negative patients had a prior survival than the other (Figure 2B,C,D).
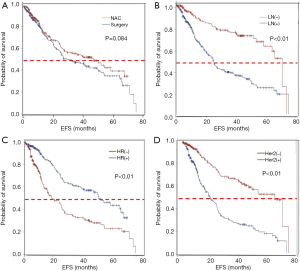
The EFS of the different subgroups was also analyzed (Figure 3). For the positive lymph node group patients, the NAC group had a better survival than the non-NAC group (Figure 3B). However, in negative lymph node patients, the difference in EFS was not significant between the NAC and non-NAC group (Figure 3A). In addition, in the HR-negative and HER2-positive group, the EFS curve showed an advantage in the NAC group over the non-NAC group (Figure 3C and

Cox proportional models were used to assess the clinicopathological factors related to prognosis (Table 3). Lymph node metastasis, HER2 status and neoadjuvant chemotherapy were found to be independent poor prognostic factors of survival in the T3 breast cancer patients. Specifically, lymph node negative, HER2-negative status, and neoadjuvant chemotherapy were associated with a longer survival time.
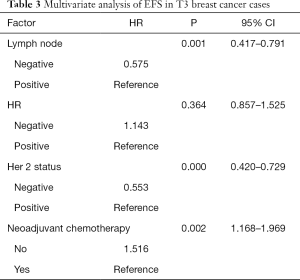
Full table
In other words, T3 breast cancer patients with positive lymph nodes and HER2-positive tumors should choose neoadjuvant chemotherapy. Meanwhile, T3 breast cancer patients with negative lymph nodes and HER2-negative tumors should choose either neoadjuvant chemotherapy or surgery as the optimal initial therapy.
Discussion
Several studies have examined the relationship between large operable or locally T3 breast cancer and survival (4). However, few investigations have compared the survival differences between NAC and non-NAC T3 patients. The present study used RWE and found that T3 patients tended to elect surgery over neoadjuvant chemotherapy as their initial therapy, and, even for those patients who chose neoadjuvant chemotherapy, the major operation was modified radical mastectomy. The inconsistency rate between imaging examination and histopathologic analysis affected the subsequent therapies. Moreover, patients with aggressive subtypes (lymph node positive, HER2 positive, HR negative) showed inferior outcome in disease-free survival (DFS) compared with their more benign counterparts. Improvements in patients who received neoadjuvant chemotherapy was evidenced by the more aggressive subtypes present in the NAC group.
Many researches have tried to explore whether imaging modalities such as magnetic resonance imaging, sonography, and mammography are as accurate at predicting breast tumor size as histopathologic analysis of resected tumors (5). However, we paid more attention to the impact of medical resources on breast cancer. Although the number of primary health-care professionals has recently increased in China, the regional distribution of doctors and medical equipment is still uneven (6). For example, only a small portion of patients received the full battery of imaging examinations for evaluating the dimension of tumor lesions. Most patients underwent biopsy or surgery after a routine ultrasound examination or even a simple physical examination. It is clear that different imaging modalities each have their respective characteristics to be used to their respective strengths, as recommended by the Chinese guidelines (7). The irrational use of imaging contributes to a high proportion of overestimation and underestimation.
At odds with the relevant literature, our study found that fewer than 5% of patients chose breast-conserving surgery (8). For those in non-NAC group, the low proportion of breast-conserving surgery was expected due to high burden of tumor lesions. Nevertheless, further discussion is required to understand how NAC leads to the downstaging of the cancer. This downstaging was initially implemented to convert inoperable patients to operable ones and later to increase rates of breast conservation in patients initially considered to be mastectomy-only candidates (9). However, we did not find these kinds of changes in this study. In some cases, this proportion may be appropriate, as patients with fewer economic resources must restrict expenditure in order to continue the expensive and prolonged therapies characteristic of T3 breast cancer treatment (3). Although it has been proven that breast-conserving surgery plus radiotherapy is as effective as mastectomy or sometimes better (10), the additional high price of radiotherapy, aesthetic considerations, and the complexity of breast-conserving technology in China all contribute to the low rate election. Moreover, no rigorous randomized clinical trials (RCTs) have yet been conducted that prove the survival benefit of breast-conserving surgery.
Although the latest guideline of the American Society of Clinical Oncology indicated no sentinel lymph node biopsy (SLNB) should be performed in large or locally advanced invasive breast cancers (11), we found that more than 10% of patients underwent SLNB, with some being clinical lymph node-positive patients. Some trials have explored the feasibility of SLNB after NAC with T0–4, N1–2, and M0 patients, and have found an acceptable false negative rate (9.8%) when combining normal axillary ultrasound with more than two SLNs removed or some other methods (12,13), although this practice is not widely accepted by experts in China. Under such circumstances, radical lymph node dissection is deemed necessary when lymph nodes are clinically positive after NAC. However, when lymph nodes are clinically negative after NAC, although SLNB appears a reasonable compromise between axillary lymph node dissection and no surgery at all, we still believe more clinical trials are needed as there is little evidence for the survival of an SLN-alone method (14).
Neoadjuvant chemotherapy, compared to conventional adjuvant therapy, does not seem to improve the overall survival of patients with breast cancer. Indeed, several RCTs have demonstrated similar outcomes, in terms of DFS and overall survival, between NAC and adjuvant chemotherapy in patients with breast cancer (15). A recent meta-analysis (16) found NAC to be associated with a higher frequency of local recurrence than that from the same chemotherapy started after surgery due to the increased breast-conserving surgery rates. Reassuringly, the increase in local recurrence was not associated with any significant increase in distant recurrence or breast cancer mortality, which indirectly supports a low rate of breast-conserving surgery in the real world. As the RCTs have provided scientific evidence for the safety and efficacy of NAC for T3 breast cancer, we used RWE to investigate the survival benefit of NAC to T3 patients (2). In our study, there was a survival benefit gained from NAC, and the results were comparable to those found in the major randomized controlled trials (17). However, patients receiving NAC were more likely to be lymph node positive with aggressive subtypes (HER2 positive HE-negative) that are the major recurrence risks for patients (18).
From this perspective, NAC does moderately reduce distant recurrence compared with to same chemotherapy given postoperatively. However, across all T3 breast cancer patients, there was no difference in EFS between the NAC group and non-NAC group. We found that NAC could also improve the survival in the T3 breast cancer patients who were lymph node-positive, and had HER2-positive or HR-negative tumors. In multivariate analysis, lymph node metastasis, HER2 status, and neoadjuvant chemotherapy were found to be an independent poor prognostic factors of survival in the T3 breast cancer patients. As the pathological and molecular features of the primary tumor are gaining importance in the decision-making process, once we find T3 breast cancer patients who are lymph node positive, HER2 positive or HR negative, we can recommend initial neoadjuvant chemotherapy rather than surgery.
There were several limitations in this study. First, we selected data when the CSCO BC database had 17,000 patient cases. However, this database has expanded to include more than 34,000 (as of January 1, 2018). Furthermore, the allure of analyzing existing data may lead to flawed conclusions, and the survival benefit might appear improved if the data pool were to be enlarged. Second, the variable regimens and courses of neoadjuvant or adjuvant therapy were not taken into consideration, which might have obscured the association between NAC and survival if different regimens meaningfully affected long-term outcomes. Third, data on pathologic complete remission was incomplete and is needed for further exploration, while the number of patients in this group was too small to draw any definitive conclusions.
The importance of NAC should be taken seriously. Our next study will involve a randomized clinical trial that compares NAC with adjuvant chemotherapy. In real world study, we will also explore the survival benefit in different molecular subtypes. Ultimately, Neither RCTs nor RWE should be overlooked, and we will combine these two methods to explore the optimal treatment of breast cancer patients.
Conclusions
For T3 breast cancer patients with positive lymph nodes, and HR-negative and HER2-positive tumors, neoadjuvant chemotherapy should be the initial treatment. Meanwhile, T3 breast cancer patients with negative lymph nodes and HER2-negative tumors should choose neoadjuvant chemotherapy or surgery as the optimal initial therapy.
Acknowledgments
Funding: The study was supported by the funding of the Chinese CSCO BC, the Natural Science Foundation of China (No. 81772845), and the Natural Science Doctoral Funding of Shandong province (no. ZR2019BH013 and ZR2017BH061)
Footnote
Reporting Checklist: The authors have completed the STROBE Checklist (available at http://dx.doi.org/10.21037/tbcr-20-21
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tbcr-20-21
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tbcr-20-21). ZJ serves as an unpaid Editor-in-Chief of Translational Breast Cancer Research. JL serves as an unpaid Managing Editor of Translational Breast Cancer Research. CG, FJ, ZF, HW serve as the unpaid editorial board members of Translational Breast Cancer Research from Mar 2020 to Feb 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. This study was approved by the Institutional Review Board of the Affiliated Hospital of Qingdao University. Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2018;29:2153. [Crossref] [PubMed]
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence - What Is It and What Can It Tell Us? N Engl J Med 2016;375:2293-7. [Crossref] [PubMed]
- Li J, Wang S, Wang Y, et al. Disparities of Trastuzumab Use in Resource-Limited or Resource-Abundant Regions and Its Survival Benefit on HER2 Positive Breast Cancer: A Real-World Study from China. Oncologist 2017;22:1333-8. [Crossref] [PubMed]
- Gillon P, Touati N, Breton-Callu C, et al. Factors predictive of locoregional recurrence following neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancer: An analysis of the EORTC 10994/BIG 1-00 study. Eur J Cancer 2017;79:226-34. [Crossref] [PubMed]
- Lai HW, Chen DR, Wu YC, et al. Comparison of the Diagnostic Accuracy of Magnetic Resonance Imaging with Sonography in the Prediction of Breast Cancer Tumor Size: A Concordance Analysis with Histopathologically Determined Tumor Size. Ann Surg Oncol 2015;22:3816-23. [Crossref] [PubMed]
- Li X, Lu J, Hu S, et al. The primary health-care system in China. Lancet 2017;390:2584-94. [Crossref] [PubMed]
- CSCO (2017). Chinese society of clinical oncology guidelines for the diagnosis and treatment of breast cancer (version 1, 2017) [M]. Beijing: People's Medical Publishing House, 2017.
- Bleicher RJ, Ruth K, Sigurdson ER, et al. Breast conservation versus mastectomy for patients with T3 primary tumors (>5 cm): A review of 5685 medicare patients. Cancer 2016;122:42-9. [Crossref] [PubMed]
- Welch HG, Prorok PC, O'Malley AJ, et al. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med 2016;375:1438-47. [Crossref] [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- yman GH, Somerfield MR, Bosserman LD, et al. Sentinel Lymph Node Biopsy for Patients With Early-Stage Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:561-4.
- Boughey JC, Ballman KV, Hunt KK, et al. Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance). J Clin Oncol 2015;33:3386-93. [Crossref] [PubMed]
- Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol. 2016;34:1072-8. [Crossref] [PubMed]
- Mocellin S, Goldin E, Marchet A, et al. Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: A systematic review and meta-analysis. Int J Cancer 2016;138:472-80. [Crossref] [PubMed]
- De Mattos-Arruda L, Shen R, Reis-Filho JS, et al. Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol 2016;13:566-79. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Korn EL, Sachs MC, McShane LM. Statistical controversies in clinical research: assessing pathologic complete response as a trial-level surrogate end point for early-stage breast cancer. Ann Oncol 2016;27:10-5. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis Lancet 2014;384:164-72. [published correction appears in Lancet. 2019 Mar 9;393(10175):986]. [Crossref] [PubMed]
Cite this article as: Liu Z, Lv M, Li J, Mao Y, Nie G, Zhang J, Geng C, Jin F, Fu P, Zha X, Fan Z, Zhang H, Jiang Z, Wang H. Neoadjuvant chemotherapy versus surgery as the initial option for T3 breast cancer (>5 cm): real-world evidence from the Chinese Society of Clinical Oncology Breast Cancer database. Transl Breast Cancer Res 2020;1:16.


