Why ACE—overview of the development of the subtype-selective histone deacetylase inhibitor chidamide in hormone receptor positive advanced breast cancer
Introduction
Breast cancer is the most frequently diagnosed cancer among women both globally and in China. In the year of 2015, there 268,600 new cases and 69,500 death with breast cancer were estimated in China (1). About three-quarters of breast cancer are hormone receptor-positive (HR+), which express estrogen receptor α (ERα) and/or progesterone receptor (PgR). Endocrine-based treatments, including selective ER modulators, selective ER down-regulators and aromatase inhibitors (AIs) are established standards of care in patients with early and advanced HR+/epidermal growth factor receptor 2-negative (HER2−) breast cancer, which have led to substantial improvements in survival outcomes for the disease population. However, over 20% of patients with early-stage disease have relapse, and almost all patients with late-stage metastatic disease yield to their illness (2,3) due to primary and acquired resistance to endocrine therapies.
Although the mechanisms of endocrine resistance in patients with HR+ breast cancer still await for fully clarified, great efforts have been made in recent years in establishing the potential link between endocrine resistance and genetic and/or epigenetic alterations (4). These include to identify the mechanisms of cross talk between ER and growth factor receptor signaling (e.g., HER family members) and survival signals PI3K/Akt/mTOR, constitutive activation of cyclin-dependent kinases (CDK) 4 and 6, and epigenetic alterations by histone deacetylases (HDACs). The above evidence has been further validated by clinical studies in patients with advanced HR+ breast cancer by combination treatment of targeted drugs with various endocrine therapies. These targeted drugs include the PI3 kinase-mTOR inhibitor everolimus (5), CDK4 and CDK6 inhibitors (palbociclib, abemaciclib and ribociclib) (6-8), the subtype-selective HDAC inhibitor tucidinostat (9) (or chidamide, as the focus of this review), and the α-specific PI3K inhibitor alpelisib (10). All these drugs have been approved and become important treatment options for patients with advanced HR+ breast cancer.
Epigenetic aberrations play important roles in disease progression and drug resistance in different types of cancer, including breast cancer (11,12). Given the fact that HDACs have a great impact on chromatin remodeling and epigenetic regulation that potentially play an important role in endocrine resistance in advanced HR+ breast cancer, HDAC inhibitors as an anti-cancer drug class have become a very interesting field of research in recent years. In the current review, the evidence from preclinical and clinical studies that links HDAC inhibitors with overcoming resistance to endocrine therapy in HR+/HER2− advanced breast cancer is highlighted. Then the review focuses on chidamide, a subtype-selective HDAC inhibitor, for its mechanisms of action, preclinical and clinical development, with a special emphasis on the ACE study that led to the first-in-disease approval of this compound in the epigenetic modulating drug class in breast cancer.
HDACs and HDAC inhibitors
Epigenetic regulation and HDACs
In general, epigenetic regulation refers to changes in gene expression and function because of alterations in chromatin structure without any changes in nucleotide sequence. Chromatin is the essential medium through which transcription factors, signaling pathways, and other stimuli alter gene expression and cellular phenotypes (11). A nucleosome, the basic unit of chromatin, comprises of an octamer of histone proteins that are wrapped with DNA. Post-translational modifications, such as acetylation, methylation and ubiquitination on specific residues of the N-terminal histone tails, result in epigenetic gene regulation. Among these modifications, acetylation and deacetylation of histone play a critical role in regulation of chromatin structure and function, thus, changes in gene expression. The acetylation and deacetylation of histones protein are controlled by two distinct enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). In general, histone acetylation results in chromatin relaxation, and gene expression is enhanced; histone deacetylation causes chromatin condensation, and gene expression is repressed (11).
In humans, at least 18 HDACs have been identified and are divided into four classes. Class I (HDAC 1, 2, 3, and 8), class II (HDAC 4, 5, 7, and 9 as IIa, and HDAC 6 and 10 as IIb), class III (SIRT 1–7), and class IV (HDAC 11) (13). Although the precise biological functions of individual HDACs are not fully identified, HDACs 1–3 (Class I) have been shown closely associated with the malignant phenotype. High expression of HDAC 1 is found in prostate, gastric, lung, esophageal, colon and breast cancers, HDAC 2 in colorectal, cervical, gastric and breast cancers, and HDAC 3 in colon and breast cancers (14,15). Increased HDAC 6 and 10 activity has also been reported to be associated with certain carcinomas (16). Predictive of poor prognosis regardless of other variables was correlated with the overexpression of these HDACs (15).
HDAC inhibitors
By targeting HDACs, HDAC inhibitors inhibit the removal of acetyl groups on histones by HDACs, which remains an active status of gene transcription and produces a comprehensive set of downstream effects, including induction of cellular differentiation and cell death, regulation of immune responses, and inhibition of angiogenesis (11). By chemical structure, HDAC inhibitors can be classified into four major classes: hydroxamates [e.g., vorinostat and belinostat], benzamides (e.g., entinostat and chidamide/tucidinostat), cyclic peptides (e.g., romidepsin) and aliphatic acids (e.g., valproic acid). Alternatively, HDAC inhibitors are also classified by their target specificity. For example, vorinostat and belinostat are pan-HDAC inhibitors, and entinostat and chidamide inhibit mainly class I HDACs (17). HDAC inhibitors that have been approved by the regulatory agency are listed in Table 1.

Full table
It should be realized that different classes of HDAC inhibitors with varying structure and target specificity could elicit distinct mechanisms of action. For instance, accumulating evidence suggests that the class I selective HDAC inhibitor entinostat, but not HDAC pan-inhibitors, is associated with a mechanism to target regulatory T cells (18) that would be potentially beneficial for the combination settings of a subtype-selective HDAC inhibitor with immunotherapy. Another important concern is the overall pharmaceutic characteristics for a drug itself. For example, to have an acceptable efficacy vs. safety profile, a drug would be required with proper pharmacokinetic and pharmacodynamic properties. Thus, selective inhibition in certain HDAC subtypes most related to cancer development with accepted pharmaceutic parameters would be advantages and crucial for an HDAC inhibitor to have an overall better efficacy and safety profile in clinical settings, particularly for solid tumors (19).
HDAC inhibitors and endocrine-resistant HR+ breast cancer
ER positive breast cancers at initial diagnosis can lose ERα expression, followed by the resistance to endocrine therapy (20). ERα repression is often related to epigenetic aberrations, or epigenetic silencing. In vitro studies have demonstrated that over-expression of HDAC 1 induces silencing of ERα gene and suppresses production of ERα protein in breast cancer cell lines (21), and HDAC inhibitors can restore the sensitivity of endocrine-resistant cell lines to endocrine treatment. For example, ER-negative breast cancer cells and AI-resistant breast cancer cells can be re-sensitized to AI treatment by the HDAC inhibitor entinostat (21) and panobinostat (22), respectively. Furthermore, HDAC inhibitors can also have effects on non-histone proteins, such as downregulation of HER2 and AKT (23), nuclear factor -kappa-B p105 subunit (NF-ĸB1) (22), and hypoxia-inducible factor 1-alpha (HIF1α) (24) in AI-resistant breast cancer cells, suggesting that posttranslational and transcriptional modulation of non-histone proteins may be also an important mechanism through which HDAC inhibitors are able to overcome endocrine resistance.
Two phase II studies of HDAC inhibitors in patients with advanced HR+ breast cancer failed to previous endocrine therapy have been reported. One was a single-arm trial to evaluate co-treatment of vorinostat (pan HDAC inhibitor) and tamoxifen in patients who had disease progression with prior endocrine therapy and chemotherapy. The results showed that in total 43 patients enrolled, 40% of them had tumor regression or stabilization of the disease by the combination treatment (25).
Another phase II trial, ENCORE 301, was a randomized, placebo-controlled study to evaluate the addition of entinostat (Class I selective HDAC inhibitor) to exemestane in patients with HR+ advanced breast cancer resistant to prior treatment with non-steroidal AI. In this trial, 134 patients were randomly assigned to exemestane plus entinostat group or exemestane plus placebo group. Combination treatment was reported to improve the progression-free survival (PFS) from 2.3 to 4.3 months and the overall survival (OS) from 19.8 to 28.1 months compared with exemestane treatment alone (26). The promising results of this study led to FDA-breakthrough approval of a pivotal phase III trial, E2112, to evaluate exemestane in combination with entinostat in patients with metastatic breast cancer having disease progression after a non-steroidal AI (27). This phase III trial, designed as co-primary efficacy endpoints of PFS and OS, enrolled 600 patients to randomly allocate by a 1:1 ratio into exemestane (25 mg, daily) and entinostat (5 mg, day 1, 8, 15, 22) group or exemestane and placebo group. However, the trial failed to meet its co-primary endpoint of PFS improvement, and the study is still going on for its final OS results (28).
Chidamide for the treatment of advanced HR+ breast cancer
Discovery and preclinical development
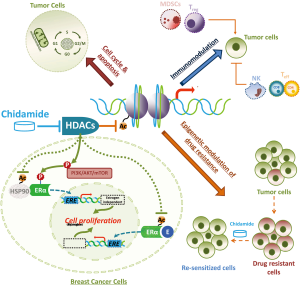
Chidamide, or by its International Nonproprietary Name (INN), tucidinostat, is a benzamide class of HDAC inhibitor with subtype specificity for HDAC 1, 2, 3 and 10 (29,30). Chidamide has been demonstrated in vitro and in vivo to induce apoptosis and growth arrest in cancer cells, reverse epithelial-mesenchymal transitions (EMT) and drug resistance of cancer cells, and enhance NK-cell and antigen-specific CD8+ T-lymphocyte-mediated antitumor activity (29-34). Apart from these general mechanisms of action, chidamide has also shown to inhibit growth factor signaling pathways, for example, to decrease phosphorylation of MEK, ERK and AKT, as well as ERα, induced by epidermal growth factor in ER+ breast cancer cells (35). Preclinical studies in animal models have verified chidamide with oral bioavailability and broad-spectrum anti-tumor activity, including breast cancers, either in single agent or in combination with other treatment regimens (29,35-37). The main proposed mechanisms of action of chidamide for its anti-tumor effects are illustrated in Figure 1.
Among preclinical studies, safety assessments of chidamide were carried out in dogs and rats with a daily dosing regimen up to 28 days. An every-three-day dosing regimen was also evaluated in rats. Animals were poor tolerated to the daily dosing, accompanied with a significant accumulation of plasma drug substance by repeat dosing. However, when chidamide was dosed in an every-three-day regimen, rats tolerated 10 times more dose exposure compared with the daily regimen. Meanwhile, no significant difference of efficacy was observed when tumor-bearing mice were dosed every day, every-other-day, or every-three-day, given the total dose-exposure was similar in a total 28-day period of time (38). Thus, the window for efficacy and tolerance was dramatically improved by interval dosing regimens, which, together with other preclinical results, provided the rationale for the design of phase I study.
First-in-human phase I and PTCL pivotal phase II studies
A phase I study was conducted to assess safety and tolerability, PK parameters and preliminary efficacy of chidamide in patients with advanced solid tumors or lymphomas (39). A total of 31 patients were enrolled and treated with oral doses of chidamide at 5, 10, 17.5, 25, 32.5, or 50 mg twice weekly (BIW cohort), or 32.5 or 50 mg three-times weekly (TIW cohort) for four consecutive weeks, followed by a two-week drug-free period. No dose-limiting toxicity (DLT) was observed in the BIW cohort up to 50 mg. DLTs were grade 3 diarrhea and vomiting in two patients in the TIW cohort at 50 mg. Generally, number and severity of adverse events (AE) increased with drug exposure. The most common AEs were fatigue (35%), thrombocytopenia (26%), anorexia (26%), leucopenia or neutropenia (23%), reduced hemoglobin (19%), nausea (16%), and diarrhea (16%). The overall safety and tolerability results suggested 50 mg BIW as the most tolerated dose (MTD), and BIW dosing regimen was recommended for further clinical studies. Chidamide PK analysis revealed its t1/2 of 16.8–18.3 h, Tmax of 1–2 h in most cases, and a dose-related increase in Cmax and AUC. Significant induction of histone H3 acetylation in peripheral white blood cells was observed after a single dose of chidamide. Out of 25 patients who had measurable lesions for preliminary efficacy assessments, 5 patients had partial response, and 4 of them were with diseases of relapsed or refractory T-cell non-Hodgkin’s lymphomas (39).
Based on the overall results from this phase I study and an exploratory phase II trial in patients with peripheral T-cell lymphoma (PTCL) who had relapsed or refractory disease (38), a pivotal phase II trial in PTCL was designed and conducted with the consent of the China FDA as an orphan drug designation. The trial was a single-arm, open-label, multicenter study of chidamide in patients with relapsed or refractory PTCL of any subtype. Patients were treated with chidamide 30 mg BIW, without drug-free holiday, until progression of the disease or unacceptable toxicity. The primary endpoint was overall response rate (ORR) as assessed by the independent review committee (IRC). A total of 83 patients were enrolled in the study and 79 patients had eligible PTCL histology for efficacy assessments. The IRC assessing results showed that 22 from 79 patients (28%) had objective response to chidamide, including 11 patients (14%) had CR/CRu. Median PFS and OS were 2.1 and 21.4 months, respectively. The most common AEs ≥ grade 3 were thrombocytopenia (22%), leucopenia (13%), and neutropenia (11%), respectively (40). Results led to China FDA approval of chidamide in relapsed or refractory PTCL in December 2014.
Why ACE
Rationale
Upon the time when chidamide was approved for PTCL indication, although HDAC inhibitors were demonstrated with the efficacy in some hematologic malignances with well acceptable safety profiles, success in any solid tumor had not been achieved either as a single agent or combined with other treatments, which brought the doubts about the therapeutic potentials for this class of drugs in solid tumors (19,41). However, several lines of evidence, particularly in breast cancer, still supported the rationale for the development of HDAC inhibitors in solid tumor indications.
First, from a point view of mechanisms of action, as demonstrated with chidamide, HDAC inhibitors have broad anti-tumor mechanisms for epigenetic aberrations presented in both tumor cell and its surrounding environment (tumor microenvironment) (42,43). These mechanisms of action are particularly supportive for the rationale of combination treatment of HDAC inhibitors with other therapies (44).
Second, HDAC inhibitors have shown promising efficacy in patients with advanced HR+ breast cancer resistant to endocrine therapies (17). Among these demonstrations, entinostat illustrated the proof-of-concept evidence in a randomized placebo-controlled phase 2 trial, in which the investigators showed that entinostat combined with exemestane had potential PFS and OS benefits in comparison with exemestane alone in patients with HR-positive advanced breast cancer resistant to prior treatment with non-steroidal AI (26).
And third, specifically to chidamide, this compound has some potential advantages in the HDAC drug class:
- Chidamide is a subtype-selective HDAC inhibitor with the inhibitory effect on class I HDAC 1, 2 and 3, and class IIb HDAC 10 (29,30), the subtypes associated with the malignant phenotype most. Since there at least 18 HDACs have been identified in humans, and some of them are very conservative for basic biological functions, selective inhibition in certain HDAC subtypes that are most related to cancer development is presumably crucial for a HDAC inhibitor to have an overall better efficacy and safety profile.
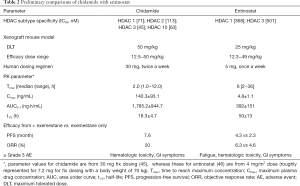
- Within the class of HDAC inhibitors, chidamide is not only an orally given drug but also appears to possess more ideal human PK properties. To demonstrate, both chidamide and entinostat are class I selective inhibitors with similar subtype selectivity and inhibitory intensity. However, under the clinical dosing regimens employed for each drug, chidamide showed a much higher exposure, and a shorter and less varied half-life than entinostat (Table 2) (45,46). These drug-structure possessed PK properties, among many other considerations, determine the dosing regimens of the two drugs currently in their clinical settings, i.e., chidamide 30 mg/twice weekly, and entinostat 5 mg/once weekly. These differences are presumably important for an anti-cancer agent to have efficacy required exposure and to improve side effect profile.
- Single agent efficacy of chidamide in PTCL with an acceptable safety profile has been demonstrated in PTCL patients. With regard to safety, chidamide is associated mainly with manageable hematologic side effects and lack of, for example, cardiotoxicity and deep vein thrombosis observed with other HDAC inhibitors (47).
Full table
Exploratory trial
A single-arm, open-label, exploratory clinical trial (45) was conducted to evaluate the safety and tolerability, PK parameters and preliminary efficacy of chidamide in combination with exemestane in postmenopausal women with advanced HR+ breast cancer who had disease relapsed or progressed from at least one previous endocrine therapy. Patients were treated with chidamide 30 mg BIW and exemestane 25 mg daily until disease progression or unacceptable toxicity.
A total of 20 patients were enrolled and received at least one dose of combination treatment. Patients had a median age of 56 years. Seven (35%) patients had visceral involvement, 14 (70%) patients had measurable disease and all remain patients had at least one mainly lytic bone lesion. Fifteen (75%) patients had received ≥3 previous therapies, and 11 (55%) patients had received at least one salvage endocrine therapy and/or salvage chemotherapy before the study entry. The median number of treatment cycle was 20.8 weeks. The most common treatment-emerged AEs ≥ grade 3 were neutropenia (35%), thrombocytopenia (30%), and leucopenia (20%). Three SAEs that could be related to chidamide treatment were a grade 3 thrombocytopenia leading to hospitalization, a grade 2 myelosuppression with poor overall conditions leading to hospitalization, and a grade 3 gastrointestinal dysfunction leading to hospitalization, respectively. The treatment continued for two patients with relieved myelosuppression after temporary treatment discontinuation or dose reduction of chidamide. For PK parameter evaluation, in brief, plasma exposures for exemestane before and after combination with chidamide were basically the same, as 32.5 vs. 31.4 ng/mL for Cmax, and 71.8 vs. 79.3 ng·h/mL for AUC0−t, respectively. For chidamide, inter- and intra-patient variations were observed in its PK parameters. A higher AUC value of chidamide after combination with exemestane (1,785 vs. 2,231 ng·h/mL) was noted, while most other PK parameters were similar before and after the combination treatment (45). Four of 16 evaluable patients were with partial response, and 10 with stable disease. The median PFS was 7.6 months (45).
Taken together, the results of this exploratory trial showed that chidamide and exemestane combination regimen was generally well tolerated with promising preliminary efficacy in advanced HR+ breast cancer patients relapsed or progressed on previous endocrine therapy, which encouraged further clinical studies of the combination treatment in this patient population.
Pivotal phase III trial
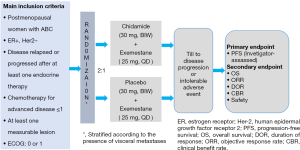
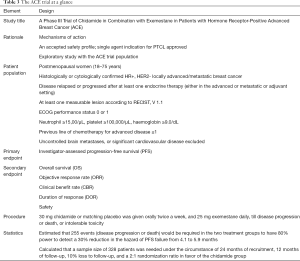
The pivotal study (ACE study) was a randomized, double blind, placebo controlled phase III trial of the AI exemestane plus chidamide/placebo in postmenopausal women with HR+ and HER2− locally advanced or metastatic breast cancer who had experienced disease relapse or progression after at least one endocrine therapy (9). Figure 2 outlines the study schema and Table 3 highlights key features of the trial.
Full table
The ACE study, led by Prof. Zefei Jiang from the 5th Medical Center of PLA General Hospital, was conducted in 22 study centers in China. Between July 2015 and June 2017, 365 patients were enrolled and randomly assigned as a 2:1 ratio into chidamide plus exemestane group (244 patients) and placebo plus exemestane group (121 patients). Due to a potential higher loss to follow-up rate was noted during the trial, 365 patients were finally enrolled, instead of the planned sample size of 328. The ACE study met its primary endpoint. Investigator-assessed median PFS was 7.4 months (95% CI: 5.5 to 9.2) with chidamide plus exemestane, and 3.8 months (95% CI: 3.7 to 5.5) with placebo plus exemestane [HR 0.75 (95% CI: 0.58–0.98); P=0.033]. In central imaging assessments by blinded independent review, median PFS was 9.2 months (95% CI: 7.2 to 10.9) in the chidamide group and 3.8 months (3.6 to 7.4) in the placebo group [HR 0.71 (95% CI: 0.53–0.96); P=0.024]. Overall response rate and clinical benefit rate were significantly higher in the chidamide group compared with that in the placebo group by both investigator and independent review assessment. Overall survival results were not mature at the date of data cutoff. Although the trial design was not powered for subgroup analysis, exploratory analysis suggested that patients with visceral disease were potentially more beneficial from the chidamide treatment (9). The most common grade 3 or 4 adverse events for combination treatment were hematological toxicities, including neutropenia [124 (51%) patients in the chidamide group vs. 3 (2%) patients in the placebo group], thrombocytopenia [67 (27%) vs. 3 (2%)], and leucopenia [46 (19%) vs. 3 (2%)]. Most hematological AEs were asymptomatic and manageable by supportive care. SAEs of any cause were observed in 51 (21%) patients in the chidamide group and 7 (6%) patients in the placebo group. No SAE occurred in more than 2% of the patients in either group.
Overall, the results of the ACE study have demonstrated that chidamide and exemestane regimen significantly improves progression-free survival and increases overall response and clinical benefit compared with exemestane alone in patients with advanced HR+/HER2− breast cancer that progressed after previous endocrine therapy. The combination of chidamide and exemestane is associated with a manageable safety profile.
Added value of the ACE study
The ACE study is the first pivotal phase III randomized trial of an HDAC inhibitor (chidamide) in combination with an endocrine therapy (exemestane) demonstrating progression-free survival benefit in patients with advanced HR+ breast cancer progressed or relapsed after previous endocrine therapy. The ACE results provide important insight into modulating epigenetic mechanism to overcome endocrine resistance, and HDAC inhibitors, as represented by chidamide, may emerge as a new treatment option in the rapidly developing landscape of targeted therapies for this common disease (48). In the meantime, the results from the ACE study have illustrated that chidamide, and probably also certain other HDAC inhibitors, is not only effective as a single agent in hematologic malignances, such as PTCL, but also an efficacious treatment in a combination way for a solid tumor. The latter would be particularly encouraging for many undergoing clinical trials using HDAC inhibitors or other epigenetic modulators in combination with various drugs with different anti-cancer mechanisms for treatment of solid tumors (44).
Results of ACE study led to the approval by China FDA of chidamide combined with an AI in postmenopausal women with HR-positive and HER2-negative locally advanced or metastatic breast cancer relapsed or progressed after previous endocrine therapy (49). CSCO (Chinese Society of Clinical Oncology) breast cancer guidelines 2020 recommends combination therapy of chidamide and exemestane as the treatment options for patients with HR+/HER2− advanced breast cancer who are endocrine-therapy naïve, failure to tamoxifen, or failure to non-steroidal AI (50).
Conclusions and future directions
During the past decade, combination of targeted therapies with established endocrine regimens has increased substantially in the treatment of endocrine resistant HR+/HER2- metastatic breast cancer, as demonstrated by the approval of mTOR, CDK4/6 and α-specific PI3K inhibitors in combination with endocrine therapy for this patient population. In addition to genetic alterations, aberrant epigenetic modification by HDACs is another important mechanism to change gene expression patterns, leading to cellular proliferation and drug-resistance in cancer. The results of the ACE study represent an important step forward in the development of HDAC inhibitors as epigenetic therapy in patients with endocrine-resistant breast cancer (48), and the chidamide combination regimen could be incorporated into clinical practice for this common disease. Meanwhile, some issues still need to be addressed in the future, such as: (I) the identification of reliable and practicable biomarkers in order to select patients who could benefit most from chidamide treatment; (II) the best treatment plans, or sequencing, when to use chidamide regimen vs. other available treatment options; and (III) whether chidamide in combination with established therapies would be beneficial for patients with HER2+ or triple-negative breast cancers.
Acknowledgments
The author thanks all the patients, physicians, nurses, and study collaborators who participated in this study.
Funding: This work was partly supported by grants from the Chinese National Major Project for New Drug Innovation (2015ZX09101005) and the Shenzhen municipal science and technology projects (JCYJ20120618162903087, JCYJ20160427185121156).
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi. org/10.21037/tbcr.2020.03.06). The author is the employee of Chipscreen Biosciences.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Davies C, Godwin J, Gray R, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patientlevel meta-analysis of randomised trials. Lancet 2011;378:771-84. [Crossref] [PubMed]
- Mauri D, Pavlidis N, Polyzos NP, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 2006;98:1285-91. [Crossref] [PubMed]
- Rugo HS, Vidula N, Ma C. Improving response to hormone therapy in breast cancer: new targets, new therapeutic options. Am Soc Clin Oncol Educ Book 2016;35:e40-54. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Jiang Z, Li W, Hu X, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:806-15. [Crossref] [PubMed]
- André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019;380:1929-40. [Crossref] [PubMed]
- Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science 2017;357:eaal2380.
- Saxena NK, Sharma D. Epigenetic reactivation of estrogen receptor: promising tools for restoring response to endocrine therapy. Mol Cell Pharmacol 2010;2:191-202. [PubMed]
- Khan AU, Krishnamurthy S. Histone modifications as key regulators of transcription. Front Biosci 2005;10:866-72. [Crossref] [PubMed]
- Barneda Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol 2012;6:579-89. [Crossref] [PubMed]
- Stimson L, La Thangue NB. Biomarkers for predicting clinical responses to HDAC inhibitors. Cancer Lett 2009;280:177-83. [Crossref] [PubMed]
- Park JH, Kim SH, Choi MC, et al. Class II histone deacetylases play pivotal roles in heat shock protein 90-mediated proteasomal degradation of vascular endothelial growth factor receptors. Biochem Biophys Res Commun 2008;368:318-22. [Crossref] [PubMed]
- Damaskos C, Garmpis N, Valsami S, et al. Histone deacetylase inhibitors: an attractive therapeutic strategy against breast cancer. Anticancer Res 2017;37:35-46. [Crossref] [PubMed]
- Shen L, Pili R. Class I histone deacetylase inhibition is a novel mechanism to target regulatory T cells in immunotherapy. Oncoimmunology 2012;1:948-50. [Crossref] [PubMed]
- Graham JS, Kaye SB, Brown R. The promises and pitfalls of epigenetic therapies in solid tumours. Eur J Cancer 2009;45:1129-36. [Crossref] [PubMed]
- Yang X, Phillips DL, Ferguson AT, et al. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res 2001;61:7025-9. [PubMed]
- Kawai H, Li H, Avraham S, et al. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int J Cancer 2003;107:353-8. [Crossref] [PubMed]
- Kubo M, Kanaya N, Petrossian K, et al. Inhibition of the proliferation of acquired aromatase inhibitor-resistant breast cancer cells by histone deacetylase inhibitor LBH589 (panobinostat). Breast Cancer Res Treat 2013;137:93-107. [Crossref] [PubMed]
- Sabnis GJ, Goloubeva OG, Kazi AA, et al. HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Mol. Cancer Ther 2013;12:2804-16. [Crossref] [PubMed]
- Naldini A, Filippi I, Cini E, et al. Downregulation of hypoxia-related responses by novel antitumor histone deacetylase inhibitors in MDAMB231 breast cancer cells. Anticancer Agents Med Chem 2012;12:407-13. [Crossref] [PubMed]
- Munster PN, Thurn KT, Thomas S, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer 2011;104:1828-35. [Crossref] [PubMed]
- Munster PN, Klein PM, Cruickshank S, et al. Randomized phase II, double-blind, placebo controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013;31:2128-35. [Crossref] [PubMed]
- Yeruva SLH, Zhao F, Miller KD, et al. E2112: randomized phase iii trial of endocrine therapy plus entinostat/placebo in patients with hormone receptor-positive advanced breast cancer. Breast Cancer 2018;4:1. [PubMed]
- Available online: http://ir.syndax.com/news-releases/news-release-details/syndax-host-conference-call-provide-update-phase-3-breast-cancer
- Ning ZQ, Li ZB, Newman MJ, et al. Chidamide (CS055/ HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol 2012;69:901-9. [Crossref] [PubMed]
- Pan DS, Yang QJ, Fu X, et al. Discovery of an orally active subtype-selective HDAC inhibitor, chidamide, as an epigenetic modulator for cancer treatment. Med Chem Comm 2014;5:1789-96. [Crossref]
- Gong K, Xie J, Yi H, et al. CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biochem J 2012;443:735-46. [Crossref] [PubMed]
- Yao Y, Zhou J, Wang L, et al. Increased PRAME-specific CTL killing of acute myeloid leukemia cells by either a novel histone deacetylase inhibitor chidamide alone or combined treatment with decitabine. PLoS One 2013;8:e70522. [Crossref] [PubMed]
- Zhou Y, Pan DS, Shan S, et al. Non-toxic dose chidamide synergistically enhances platinum-induced DNA damage responses and apoptosis in non-small-cell lung cancer cells. Biomed Pharmacother 2014;68:483-91. [Crossref] [PubMed]
- Lu X, Ning Z, LI Z, et al. Discovery and development of HDAC subtype selective inhibitor chidamide: potential immunomodulatory activity against cancers. In: Fischer J, Childers WE. Editors. Successful Drug Discovery. Wiley-VCH Verlag GmbH & Co. KGaA, 2016:89-108.
- Zhou Y, Wang YN, Zhang K, et al. Chidamide reverses epidermal growth factor induced endocrine resistance in estrogen receptor-positive breast cancer. J Shenzhen Univ Sci Eng 2018;35:339-44. [Crossref]
- Liu L, Qiu S, Liu Y, et al. Chidamide and 5-flurouracil show a synergistic antitumor effect on human colon cancer xenografts in nude mice. Neoplasma 2016;63:193-200. [PubMed]
- Zhang N, Liang C, Song W, et al. Antitumor activity of histone deacetylase inhibitor chidamide alone or in combination with epidermal growth factor receptor tyrosine kinase inhibitor icotinib in NSCLC. J Cancer 2019;10:1275-87. [Crossref] [PubMed]
- Lu X, Ning Z, Li Z, et al. Development of chidamide for peripheral T-cell lymphoma, the first orphan drug approved in China. Intractable Rare Dis Res 2016;5:185-91. [Crossref] [PubMed]
- Dong M, Ning ZQ, Xing PY, et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol 2012;69:1413-22. [Crossref] [PubMed]
- Shi Y, Dong M, Hong X, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol 2015;26:1766-71. [Crossref] [PubMed]
- Nolan L, Johnson PW, Ganesan A, et al. Will histone deacetylase inhibitors require combination with other agents to fulfil therapeutic potential? Br J Cancer 2008;99:689-94. [Crossref] [PubMed]
- Azad N, Zahnow CA, Rudin CM, et al. The future of epigenetic therapy in solid tumours—lessons from the past. Nat Rev Clin Oncol 2013;10:256-66. [Crossref] [PubMed]
- Jones PA, Issa JPJ, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet 2016;17:630-41. [Crossref] [PubMed]
- Morel D, Jeffery D, Aspeslagh S, et al. Combining epigenetic drugs with other therapies for solid tumours —past lessons and future promise. Nat Rev Clin Oncol 2020;17:91-107. [Crossref] [PubMed]
- Zhang Q, Wang T, Geng C, et al. Exploratory clinical study of chidamide, an oral subtype-selective histone deacetylase inhibitor, in combination with exemestane in hormone receptor-positive advanced breast cancer. Chin J Cancer Res 2018;30:605-12. [Crossref] [PubMed]
- Gore L, Rothenberg ML, O’Bryant CL, et al. A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin Cancer Res 2008;14:4517-25. [Crossref] [PubMed]
- Shah RR. Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Safety 2019;42:235-45. [Crossref] [PubMed]
- Wander SA, Spring LM, Bardia A. Genetics to epigenetics: targeting histone deacetylases in hormone receptor-positive metastatic breast cancer. Lancet Oncol 2019;20:746-8. [Crossref] [PubMed]
- Available online: https://www.chipscreen.com/en/index.php/show-626.html
- CSCO BC. Chinese society of clinical oncology guidelines for diagnosis and treatment of breast cancer (version 2020). People's Medical Publishing House, 2020.
Cite this article as: Ning Z. Why ACE—overview of the development of the subtype-selective histone deacetylase inhibitor chidamide in hormone receptor positive advanced breast cancer. Transl Breast Cancer Res 2020;1:5.